What is water hardness
Hydrogen oxide (water) is the most common liquid on earth, which has the unique properties of dissolving many chemical elements and compounds. The concept of water hardness in the definition is interpreted as a quantitative indicator of the concentration of salts of alkaline earth and other metals. These are mainly calcium and magnesium compounds.
The term general water hardness arose as a result of observations of the condition of fabrics after washing. The special structure of the material, consisting of individual threads, contributes to the accumulation of the mentioned metal salts. With a large content of them, the fabric becomes hard and rough. Later it was noticed that high concentrations of such compounds significantly reduce the effectiveness of detergents.
Water hardness is calcium and magnesium salts that form scale on the internal surfaces of heating devices and heat exchangers. This in turn leads to a deterioration in the operational and economic characteristics of heating equipment. In some cases, pipelines are literally clogged, making it impossible to use them for their intended purpose. You can determine whether there is hardness in water even at home!
Ways to soften water
There are simple ways to soften water at home with your own hands. To select a method, you need to decide for what purpose the water will be prepared - for bathing children, washing, cooking, for an aquarium, or for other household needs.
To soften water in large volumes, it is recommended to install special devices - water softening filters with an insertion into the water supply system or functioning independently.
It is possible to reduce the level of water hardness at home in the following ways:
- Standing is a simple method to perform. The water tank is placed aside for 1-2 days, away from the sun's rays. Settled water is often used for watering indoor and garden plants. Replenishment of water for these needs is carried out from wells or wells.
- Boiling is the simplest method of purifying water from hard compounds without the use of chemicals and organics. Heating to high temperatures affects the decomposition of salts and leads to their settling on the bottom of the dish. In this simple method, the beneficial properties of drinking water are lost, energy consumption increases, and scale will have to be removed.
- Freezing - this method is based on partial freezing of a hard liquid. The water is poured into a container of the required dimensions and installed in the cooling chamber. When an ice ball forms on the surface of the tank, the remaining water is poured out. The resulting ice, when thawed, will become suitable for drinking. Useful qualities will not be lost, but bad impurities will leave.
The main reasons for the formation of water hardness
In fact, this liquid is a universal solvent, which, upon contact with soil and minerals, is saturated with the substances and chemical compounds it contains. Answering the question about water hardness and what causes it, it is necessary to highlight the main sources of alkaline earth metal salts:
- For underground springs, these are layers of limestone through which liquid seeps.
- For open reservoirs and near-surface layers, these are geological rocks: natural gypsum, dolomite and others.
- Calcium and magnesium ions saturate the water as a result of chemical reactions between carbon dioxide and certain minerals.
- Microbiological processes in soils and agricultural lands in catchment areas.
In addition to natural causes, the increased content of hardness ions in water is also due to man-made ones. The wastewater from industrial, construction and municipal enterprises contains a large amount of impurities. As a result, secondary pollution of water bodies and aquifers occurs.
Natural processes of chemical weathering of rocks and man-made factors determine the hardness parameters of drinking or technical water. These indicators are not the same for different sources and are constantly changing:
- They increase as a result of the evaporation of moisture from the open surfaces of reservoirs.
- They decrease with precipitation, melting ice and snow.
A significant content of lime in water is a hardness of predominantly natural origin, characteristic of underground sources, including artesian wells. The mineral is washed out of geological layers by liquid flows when seeping deep into the earth's crust. Then this solution, through springs and springs, replenishes the water balance of open sources.
There is a constant circulation, and the hardness of the water depends to some extent on the time of year. In the spring, during the melting of snow and active precipitation, it decreases significantly in other periods and, especially during droughts, increases.
Why soften water
For different regions, hard water is a common feature. Many people resort to the need to soften water. The plaque that forms on the bottom of the kettle indicates a problem with water hardness.
This is often discovered when using water from an artesian well, because the level of water hardness is mainly determined by the content of calcium and magnesium in it. These mineral compounds create carbonate hardness in different waters.
- It is possible to soften a liquid with temporary hardness in 1 hour by resorting to the boiling procedure. Carbonate from the walls will sink to the bottom of the dish in the form of a whitish sediment.
- However, if water with a temporary liquid can be softened by boiling, then this method does not work for permanent liquid formed by sulfate and calcium salts. These salts will not be able to dissolve.
- The high hardness of drinking water gives it a bitter taste, and its consumption can cause damage to health. It can cause disruptions in the functioning of the stomach and intestines.
- In domestic conditions, such water will lead to damage to washing and dishwashing machines, boilers, and kettles. A negative effect on plumbing pipes has also been noticed; they become clogged and quickly become unusable under the influence of excessively hard water.
Noting all of the above, it is worth softening the level of water hardness, namely, reducing the calcium-magnesium compounds in it.
Types of water hardness
The level of salt content and their composition for different water supply sources, both open and closed, differ significantly. A natural question arises: what is the hardness of water, and what it can be depending on its origin. There is a classification according to the level of saturation with chemicals and compounds, summarized in a table for convenience:
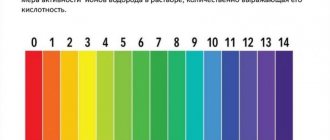
| Name | mg∙eq/l | Degrees °dH | ppm |
| Very soft | no more than 1.5 | 0-4° | 0-70 |
| Soft | from 1.5 to 4.0 | 5-8° | 70-140 |
| Medium hardness | from 4.0 to 8.0 | 9-12° | 140-210 |
| Tough | from 8.0 to 12.0 | 13-22° | 210-320 |
| Very tough | over 12.0 | 23-34° | 320-530 |
The above scale clearly shows the levels of water hardness and how it is measured in systems adopted in different countries. This classification reflects methodological approaches to determining the indicated indicator in accordance with the regulatory documents of the following countries:
- Russia. State standard.
- Germany. Standardization Institute - DIN.
- USA. United States Environmental Protection Agency - USERA.
The mentioned degree of water hardness is a unit of measurement adopted in Western countries. This indicator corresponds to the following values:
- In Germany (°dH). One part CaO or 0.719 parts MgO per 100 thousand parts of water.
- In Britain (°e). 1 grain CaCO3 per gallon of liquid.
- In the USA (ppm) and France (°TH). One part CaCO3 per 100 thousand parts water.
With a relatively low level of water hardness, the content of calcium ions in it can reach 70-80%, while at the same time the saturation with magnesium ions rarely exceeds 50-60%. With an increase in the level of mineralization, the picture changes dramatically: the concentration of the former decreases greatly and rarely reaches 1 g/l; the content of magnesium ions often exceeds 10 g/l. This ratio is especially typical for salt lakes that have no drainage.
Classification of water is carried out not only by the level of hardness, but also taking into account its hydrochemical composition. Based on this characteristic, the following varieties are distinguished:
- General.
- Temporary (carbonate).
- Permanent (not carbonate).
Each type is characterized by a certain ratio of calcium, magnesium and other salts. Accordingly, methods for reducing water hardness depend on its type, and the composition of the equipment for a particular case is determined taking into account real indicators. Let's consider this issue in more detail.
Total water hardness
The studied indicator largely depends on the hydrochemical composition of the liquid and the level of saturation with calcium and magnesium salts. Total hardness is a property of water determined by the total content of bicarbonates and other compounds of these elements. This characteristic has two components:
- Carbonate (temporary). It is associated with the presence of calcium (Ca2+) and magnesium (Mg2+) bicarbonates in the liquid, which, when heated and boiled, form CaCO3 and Mg(OH)2 with the release of carbon dioxide (CO2).
- Non-carbonate (permanent). Determined by the presence of phosphates, chlorides, sulfates, silicates and nitrates of these alkaline earth metals.
The listed salts, which determine the overall hardness of water, do not decompose when boiled and their removal from the liquid requires other methods. To express this indicator numerically, a special physical unit of 1 mmol-eq/l is used, which corresponds to the content of 20.04 mg/l calcium cations or 12.16 mg/l magnesium cations.
The total hardness of groundwater (in some regions), as well as sea and ocean water, can reach 80 and even 100 mmol-eq/l, which makes them unsuitable for use. At the same time, in rivers and lakes located in taiga regions, this indicator is at a level of 0.1 to 0.2 mmol-eq/l. It is almost impossible to wash off the soap solution with such water.
Temporary hardness (carbonate)
Natural water contains a significant amount of alkaline earth metal cations. Temporary (magnesium and calcium) water hardness is an indicator of the presence of hydrocarbonates of the named chemical elements in the liquid. When heated, the solubility of these compounds (at a pH level exceeding 8.3 units) decreases significantly, and they transform into the following forms:
- flocculent sediment;
- whitish film on the surface;
- crystalline scale.
Temporary water hardness in terms of calcium and magnesium is completely eliminated - hence the name. In addition to boiling, it is possible to reduce this indicator using ion exchange methods and reverse osmosis technologies.
Constant hardness (non-carbonate) water
In addition to calcium and magnesium bicarbonates, water supplies contain significant quantities of salts of nitric, sulfuric and hydrochloric acid. Non-carbonate water hardness refers to the total content of these chemical compounds. When heated, the latter remain in a dissolved state and do not precipitate.
This is why non-carbonate hardness of water is called permanent, and special equipment is required to eliminate it. It is possible to significantly reduce the concentration of these soluble compounds using ion exchange technologies and demineralization. Modern softening systems are especially efficient.
The difference between temporary and permanent rigidity
Temporary hardness is an admixture of magnesium and calcium compounds in tap water, which are called bicarbonates and carbonates. When carrying out the procedure of boiling this water, the compounds disintegrate, thereby settling on the walls of the kettle in the form of scale. It turns out that when boiling, the hardness eliminates itself, which is why it is called temporary.
Constant hardness - when the presence of magnesium and calcium salts in water form compounds with strong acids, such as nitric, sulfuric or hydrochloric. No matter how hard housewives boil the kettle, these impurities in the water after boiling will not disappear anywhere.
Temporary and permanent hardness in other sources may be referred to as carbonate and non-carbonate water hardness, respectively. The same water can have two of these characteristics at once, and their sum is called “total hardness”.
Current water hardness standards
Severity standards characterizing the quality of water supply sources are regulated in all developed countries. The Russian Federation has adopted GOST 31954-2012, which sets limit values for total water hardness and determines methods for calculating it. This document approves two methods: complexometric - basic and atomic spectrometry - arbitrary.
The total water hardness in terms of hygienic standards is established by the requirements of SanPiN 2.1.4.1075-01, approved by the Resolution of September 26, 2001. The total content of alkaline earth metal salts should not exceed the maximum permissible concentrations, which are defined as 7.0 and 10.0 mg-eq/l. The second value can be set only by decision of the chief sanitary doctor, taking into account the water treatment technology used for a particular locality.
CONTENT
- 1 Origin
- 2 Permanent hardness 2.1 Temporary hardness
- 3.1 Softening
- 4.1 Hard/soft classification
- 5.1 In Australia
Calculation of water hardness
When determining the total hardness of water, the use of computational methods is allowed. This indicator is measured in mmol/dm3 or mol/m3 and is described by the following formula:
JO = [Ca2+] + [Mg2+] = FA + JNA;
This equation allows you to calculate the average hardness of water; it uses the following notation:
JO - general.
LC - carbonate (temporary).
ZhNK - non-carbonate (permanent).
In order to calculate the total hardness of water, it is necessary to know the molar mass of calcium and magnesium cations, as well as sulfurous acid anions. The results of the calculations can be used to determine the most appropriate cleaning method. Such calculations are carried out by specialists from specialized laboratories based on experimental data.
Impact on human health:
How does hard water our body? Doctors associate the appearance of urolithiasis with this particular feature of water. But at the moment there is no official confirmation of this hypothesis. But we know that too hard water adversely affects the organoleptic properties of water, giving it a bitter taste. Also, hard water dries out our skin and hair. However, it is not recommended to completely switch to soft water. Our body needs calcium and magnesium salts to strengthen the cardiovascular system. But it is better to water indoor plants with soft or melt water.
Methods for determining water hardness level
How to determine water hardness? An accurate assessment of the quantitative parameters of salt content is carried out by specialized laboratories. In accordance with GOST 31954-2012, the total hardness of water is determined by the complexometric method in the following sequence:
- The sample is divided into two equal parts.
- 100 ml of the test water is poured into a chemical flask, 5 ml of a buffer solution and a dry indicator mixture in an amount of 0.06-0.10 g are added to it. The resulting composition is titrated with Trilon B.
- Similar actions are performed for the second part of the sample in another laboratory vessel. Trilon B is added in two steps: first, 0.5 cm3 less than when studying the first sample. After thorough mixing, the remainder is added.
Processing of the results obtained using the described method for determining the total hardness of water is carried out according to the following formula:
F = M•K•Vтp/Vnp,
where M is the conversion factor;
K—correction factor;
Vtp is the volume of Trilon B used for titration;
Vnp is the amount of water being tested from the sample.
The test result according to this method for determining the total hardness in drinking water, as well as technical water, is calculated as the arithmetic mean of the two obtained values. This allows us to minimize calculation errors.
Technical devices
The use of technology reduces time consumption and makes it possible to purify large volumes of liquid.
- Plate filter. The device is built on a non-chemical method of purifying moisture. Installation of a filter in the form of double-sided magnets is carried out on different water pipes. With the help of the resulting magnetic field, salt particles are retained and sent to settling tanks. The water is softened, but its quality does not decrease.
- Filter jug. It gained popularity due to its inexpensive cost and simple device. A replaceable carbon-filled cartridge serves as a barrier to harmful substances. Depending on the frequency of use and water hardness, the filter will need to be changed every two months.
- Device with ion exchange resin. The installation connected to the water supply system contains two containers. In the first tank, the water is purified with resins, and when moving to the second, it is softened with a saline solution. From time to time, the chemical components of the filter need to be replaced, and the unit needs to be connected to the sewer system.
- Filter with membranes. Provides the best results in softening liquids. The water in the device passes under pressure through the filter membranes. Together with softening, the liquid is cleansed of negative bacteria. As a result of specific cleansing, beneficial substances are also removed along with harmful substances. The filter device is expensive and cannot clean large amounts of liquid.
In some cases, you can soften small amounts of liquid using traditional methods. To permanently reduce hardness in large quantities, you will need to purchase and connect special filters to the system.
Household methods of water softening
High salinity and hardness of water used for food and household purposes can be harmful to health and lead to failure of household appliances. At home, this figure can be reduced by thermal and chemical treatment of the source liquid.
Removable water hardness - carbonate (temporary) decreases with prolonged boiling. During the heating process, calcium and magnesium bicarbonate compounds disintegrate and turn into a solid state. They form scale on the walls of dishes, flaky sediment and surface deposits. The latter are easily removed by filtering or settling.
Chemical treatment to reduce the hardness of the source water is carried out using lime and soda. The first reagent provides softening of a liquid with a low content of non-carbonate compounds and a high saturation with carbonate compounds. To increase the efficiency of this method, coagulant reagents are additionally used.
Combining lime and baking soda allows you to reduce the total hardness of river or well water to 1.4-1.8 mg eq/l. Chemical treatment improves the quality of the liquid in terms of this indicator, but requires a very precise dosage of reagents. In addition, it is unsuitable for cooking and drinking.
Links[edit]
- "Hard water". National Ground Water Association. Retrieved June 28, 2021.
- ^ abc World Health Organization Drinking Water Hardness, 2003
- ^ abc Weingärtner, Herman] (December 2006). Ullman's Encyclopedia of Industrial Chemistry - Water
. Weinheim: Wiley–VCH. DOI: 10.1002/14356007.a28_001. - "Map showing hardness levels in mg/l as calcium carbonate in England and Wales" (PDF). DEFRA / Drinking Water Inspection. 2009
- ^ abc USGS - United States Geological Survey Office of Water Quality. "USGS Water Quality Information: Water Hardness and Alkalinity". usgs.gov
. - Christian Nitsch, Hans-Joachim Heitland Horst Marsen, Hans-Joachim Schlüussler, "cleaning agents" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. DOI: 10.1002/14356007.a07_137
- Sengupta, Pallav (August 2013). "The Potential Health Effects of Hard Water". International Journal of Preventive Medicine
.
4
(8):866–875. ISSN 2008-7802. PMC 3775162. PMID 24049611. - "Lime softening". Retrieved November 4, 2011.
- Wisconisin DNR - Carbonate Chemistry
- ↑
Stephen Lower (July 2007). "Hard water and water softening". Retrieved October 8, 2007. - P. P. Coetzee (1998). "Zn-induced downscaling and scale modification effects" (PDF). Archived from the original (PDF) on May 09, 2021. Retrieved March 29, 2010.
- Sorg, Thomas J.; Schock, Michael R.; Little, Darren A. (August 1999). "Ion exchange softening: effects on metal concentrations". AWWA Magazine
.
91
(8):85–97. DOI: 10.1002/j.1551-8833.1999.tb08685.x. ISSN 1551-8833. Archived from the original on 2011-07-26. Retrieved November 23, 2010. - "Drinking water, water hardness, calcium, magnesium, dyed laundry." Water-research.net. Retrieved January 26, 2013.
- František Kožíšek Health Importance of Drinking Water Calcium and Magnesium Archived 2013-04-18 at the Wayback Machine, February 2003
- ↑
Pocock SJ, Shaper AG, Packham RF (April 1981).
"Research on water quality and cardiovascular disease in the United Kingdom." Sci. Total Environ
.
18
: 25–34. Bibcode: 1981ScTEn..18…25P. DOI: 10.1016/S0048-9697(81)80047-2. PMID 7233165. - Marque S, Jacqmin-Gadda N, Dartigues DF, Commenges D (2003). "Cardiovascular disease mortality and calcium and magnesium levels in drinking water: an ecological study in older adults" (PDF). Euro.
J. Epidemiol .
18
(4): 305–9. DOI: 10.1023/A: 1023618728056. PMID 12803370. S2CID 1834547. - Rubenowitz E, Axelson G, Rylander R (January 1999). "Magnesium and calcium in drinking water and death in women from acute myocardial infarction." Epidemiology
.
10
(1): 31–6. DOI: 10.1097/00001648-199901000-00007. PMID 9888277. - ↑
McNally NJ, Williams HC, Phillips DR, Smallman-Raynor M, Lewis S, Venn A, Britton J (1998).
"Atopic eczema and household water hardness". Lancet
.
352
(9127):527–531. DOI: 10.1016/S0140-6736 (98) 01402-0. PMID 9716057. S2CID 6319959. - ↑
Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T (January 2004).
"Environmental association of water hardness with the prevalence of childhood atopic dermatitis in urban Japan." Environ.
Res .
94
(1):33–7. Bibcode: 2004ER…..94…33M. DOI: 10.1016/S0013-9351(03)00068-9. PMID 14643284. - Arnedo-Pena A, Bellido-Blasco J, Puig-Barbera J, Artero-Civera A, Campos-Cruanes JB, Pac-Sa MR, Villamarin-Vazquez JL, Feliz-Dauder C (2007). "Domestic water hardness and the prevalence of atopic eczema in schoolchildren in Castellón (Spain)". Salud Publica de Mexico
.
49
(4):295–301. DOI: 10.1590/S0036-36342007000400009. PMID 17710278. - Perkin MR, Craven J, Logan K, Strachan D, Marrs T, Radulovic S, Campbell LE, McCallum SF, McLean WH, Luck G, Flor S "Association between household water hardness, chlorine content and risk of atopic dermatitis in early life: a population-based cross-sectional study" (PDF). J Allergy Clin Immunol
.
138
(2):509–516. DOI: 10.1016/j.jaci.2016.03.031. PMID 27241890. - Multicentre randomized controlled trial of ion exchange water softeners for the treatment of eczema in children: Softened Water Eczema Trial (SWET) protocol (ISRCTN: 71423189) https://www.swet-trial.co.uk/
- "Hardness of water" . thekrib.com
. - Total water hardness [ permanent dead link
] - ↑
Water corrosion. Archived October 20, 2007, at the Wayback Machine. - ^ ab McTigue, Nancy E.; Simons, James M., ed. (2011). Aquatic Dictionary: A Comprehensive Guide to Aquatic Terminology. American Water Works Association. pp. 333–. ISBN 978-1-61300-101-1.
- ^ab Reed, Robert N. (2003). Water Quality Systems: A Guide for Facilities Managers. CRC Press. P. 66–. ISBN 978-0-8247-4010-8.
- Langelier, W. F. (October 1936). “Analytical control of anti-corrosion treatment of water.” Journal of the American Water Works Association
.
28
(10): 1500–1521. DOI: 10.1002/j.1551-8833.1936.tb13785.x. JSTOR 41226418. - Aquaprox, ed. (2009). Cooling water treatment. Springer. P. 104–. ISBN 978-3-642-01985-2.
- Emerson A. G. D. (2003). Quantitative Forecasting of Problems in Industrial Water Systems. World Scientific. P. 7–. ISBN 978-981-238-184-2.
- Ryznar, John W.; Langelier, W. F. (April 1944). "A new index for determining the amount of calcium carbonate deposits formed by water." Journal of the American Water Works Association
.
36
(4):472–486. DOI: 10.1002/j.1551-8833.1944.tb20016.x. JSTOR 23345279. - T.E., Larson and R.W. Skold, Laboratory Studies Relating to Mineral Water Quality and Corrosion of Steel and Cast Iron, Illinois State Water Survey, 1958, Champaign, Illinois, pp. [43] - 46: ill. ISWS C-71
- Stiff, Jr., H. A., Davis, L. E., A Method for Predicting the Tendency of Oil Field Waters to Precipitate Calcium Carbonate, Pet. Per. AIME 195; 213 (1952).
- Oddo, J.E., Thomson, M.B., Scaling Control, Prediction and Treatment, or How Companies Evaluate the Scaling Problem and What They're Doing Wrong, CORROSION/92, Paper No. 34 (Houston, TX: NACE INTERNATIONAL 1992). KK
- "Dishwasher and water hardness - Water quality in Canberra - About us". actewagl.com.au
. Archived from the original on 2012-03-26. - melbournewater.com.au
(PDF) https://web.archive.org/web/20130511163534/https://www.melbournewater.com.au/content/library/publications/reports/compliance_reports/public_health_compliance_quarter_1_(july-september_2006) .pdf. Archived from the original (PDF) on 2013-05-11. Retrieved December 17, 2006. Missing or empty |title=( help ) - "Sydney typical drinking water analysis". Archived from the original on 2013-01-16. Retrieved December 17, 2006.
- "WA Water Corporation - 404" (PDF). watercorporation.com.au
. Archived from the original (PDF) on September 4, 2007. - "Brisbane's drinking water". Archived from the original on 2007-11-02. Retrieved December 17, 2006.
- "Adelaide Water Quality". Archived from the original on 2013-03-15. Retrieved November 30, 2012.
- "Hobart City Council, Tasmania, Australia". hobartcity.com.au
. Archived from the original on 2008-02-10. - "Darwin Water Quality" (PDF). Archived from the original (PDF) on September 30, 2007. Retrieved December 17, 2006.
- "Ville de Montréal - L'eau de Montréal". .ville.montreal.qc.ca. 2013-01-22. Retrieved January 26, 2013.
- Canadian Water Quality Association. "Water Hardness/Total Number of Households in Canadian Cities" (PDF). Archived from the original (PDF) on October 4, 2013. Retrieved October 4, 2013.
- "FAQ" . Saskatoon.ca. Retrieved January 26, 2013.
- Winnipeg drinking water quality test results, 2006
- "Water - Services - Life in Toronto - City of Toronto". toronto.ca
. 2017-07-14. - GVRD Wash Smart - Water Facts
- "CITY OF CHARLOTOWN WATER SUPPLY AND SEWER 2006 Water Report" (PDF). Aquasafecanada.com. Retrieved January 26, 2013. [ permanent dead link
] - "REGION OF WATERLOO Residential Water Softener Efficiency Test Report No. 1, April 2011." (PDF). regionofwaterloo.ca. Archived from the original (PDF) on 10/13/2017. Retrieved January 26, 2013.
- “Notice to the Public: West Side Water Supply | Saint John". www.saintjohn.ca
. Retrieved October 10, 2021. - Department of Public Works and Environmental. ottawa.ca
. Retrieved June 19, 2021. - "Table 2 Hardness of drinking water". United Utilities
. Archived from the original on 2012-04-13. Retrieved March 3, 2012. - ^ ab "Drinking water quality". United Utilities
. Retrieved March 3, 2012. - "Severn Trent Water - B1 1DB". Severn Trent Water
. Archived from the original on 2012-05-03. Retrieved March 3, 2012. - "Water hardness levels in Bristol". Bristol water
. Archived from the original on 2011-08-01. Retrieved March 3, 2012. - "Southern Water - Zone SO14". South water
. Archived from the original on 2012-11-23. Retrieved March 3, 2012. - "EC1A 7BE - Water quality in your area". Thames water
. Archived from the original on 2012-05-27. Retrieved March 3, 2012. - dwi.gov.uk
- anglianwater.co.uk
- Section 36 Hardness https://www.epa.ie/pubs/advice/water/quality/Water_Quality.pdf
- Wilson, Amber; Parrott, Kathleen; Ross, Blake (June 1999). "Water quality at home - water hardness". Retrieved April 26, 2009.
- Briggs, J.C., and Fike, J.F.; U.S. Stream Quality, Water Year 1975 - Based on the National Stream Quality Accounting Network (NASQAN)
: USGS Open Access Report 78-200, 436 pp. (1977) - “Is there hard water? Here's what you need to know about it." The pulse of the modern home. 2018-01-22. Retrieved September 22, 2021.
Professional methods for reducing water hardness
High-quality softening of source water to the required level is only possible with the use of special equipment - water hardness filters. The following technical devices allow the hardness indicators to comply with the requirements of the mentioned SanPiN and GOST drinking water:
- Softening filters.
- Ion exchange filters.
- Reverse osmosis systems.
When choosing a device to reduce water hardness, a device for determining it determines the exact hydrochemical composition and quantitative salt content. Equipment is selected based on test results and taking into account customer requirements for the quality of treated water.
Softening filters
The process of reducing the overall hardness of water should be quite effective and inexpensive. Currently, the main method of softening is the sodium cationization method (softening filters), which is used both in everyday life and on an industrial scale. The water flow is passed through ion exchange columns, where calcium and magnesium are replaced by sodium cations. The following chemical reactions occur on the surface of polymer resins:
Ca2+ + 2RNa = 2Na+ + R2Ca
Mg2+ + 2RNa = 2Na+ + R2Mg
As a result, the total hardness is brought to the level of drinking water according to GOST, which makes it possible to use it for cooking and household needs.
Such filter columns are used as part of multi-stage systems in which water is first purified from mechanical impurities and iron. This allows you to increase the resource of the main cartridges, which naturally decreases in the process of consumption of sodium ions and accumulation of hardness ions. To restore the properties of ion exchange resins, the system includes tanks with a solution of table salt, valves and control units.
When the sensor detects an increase in water hardness in ppm, its supply is shut off and the contents of the column are backwashed with saline solution. After the characteristics of the polymer granules are restored, the supply of the reagent is stopped. The valve opens and the softening process resumes.
The operation of such a filter, which reduces the overall hardness of natural water, is controlled automatically or manually, depending on the complete set. The first option uses electronic sensors and electrovalves. In the second case, switching the installation to regeneration mode is performed by a person after a certain period of time.
Ion exchange filters
This softening method is largely similar in principle to that described above. The replacement of ions that cause water hardness occurs using multicomponent resins such as Ecomix or Ecotar. These filter media contain the following materials:
- Inert resin ensures deferrization of water.
- FerroSorb removes iron and manganese compounds.
- HumiSorb neutralization of organic impurities.
- Ion exchange resin for water softening.
- Quartz sand in the form of a substrate that ensures uniform distribution of liquid flows.
The use of multicomponent compositions for filter installations makes it possible to reduce the overall hardness of water due to the substitution reaction of calcium and magnesium cations. This technology is more universal in comparison with softening filters, however, it is also less effective. At the same time, the financial costs for its acquisition and maintenance are higher than for specialized elements.
Reverse osmosis
The use of semi-permeable membranes ensures the highest level of liquid purification from all types of impurities. In modern reverse osmosis systems, the total hardness of water after conditioning on membranes such as DRO-4040 or DRO-8040 is reduced by more than an order of magnitude. Such installations are used for desalination of salt water (ocean, sea, lake and underground).
The membranes have a porous structure that allows only water molecules to pass through and retains calcium and magnesium compounds (bicarbonate and acid salts). As a result, the total hardness of water is reduced to values that make it possible to use it in pharmaceuticals and microelectronics. Permeate contains virtually no microelements and requires mineralization for drinking and cooking.
Such indicators, which significantly exceed GOST for drinking water in terms of overall hardness, are clearly excessive. Taking into account the high initial costs of purchase, installation and subsequent maintenance, it is not economically profitable to use reverse osmosis plants in everyday life. It is much more effective to use traditional softening filters for such purposes.
How to determine water hardness using special instruments
Water must have an optimal hardness level, since the complete absence of salts is harmful to the human body. If, for example, there are few carbonate salts in the water, this leads to the appearance of cardiovascular diseases.
If we talk about the containers themselves in which water is heated, then soft water promotes corrosion. Therefore, after operating equipment with soft water in thermal power engineering, the surfaces are additionally treated with a solution containing substances that slow down this process.
Thus, water, no matter what source you take it from, has some kind of hardness indicator, and it is ideal if it has an average value, because both an excess of salts and a lack of them lead to certain consequences.
Determining the hardness of water in our time is not difficult - now you can purchase special devices to control this indicator both at home and at work. The best option is the TDS-3 device; it can be found on the market under different trade names.
You can use another device that allows you to determine the hardness of water - an electrolyzer. This is a fairly cheap device.
The Electorlizer will not be able to determine the level of water hardness in numbers, but it will color the liquid a certain color depending on how much salt it contains. After electrolysis, you will also understand which impurities you are dealing with by the color of the water.
You can make such a device yourself; to do this, take:
- stainless steel, size 50 x 50 cm;
- bolts M6 x 150;
- washers;
- nuts;
- transparent tube;
- fittings;
- plastic container with a volume of one and a half liters;
- filter for water purification;
- check valve for water.
The basis for the future device will be a stainless steel sheet, ideally AISI 316L if it is imported, and 03Х16Н15М3 if it is domestic.
Now the stainless steel needs to be marked and cut into 16 identical squares, after which we cut off one corner of each of them, and drill a hole on the opposite corner, which will come in handy a little later. The principle of operation of the electrolyzer is the movement of electricity from a plate of one charge to a plate of the opposite charge, as a result - water breaks down into oxygen and hydrogen.
In order to create a good flow of current, the plates are connected alternately: first a positive charge, then a negative charge, then a positive charge again, etc. The tube is used for insulation; a ring needs to be cut from it, cutting which we get a strip 1 mm thick.
Washers are needed to assemble the plates: screw a washer onto a bolt, then a plate and three washers, then a plate again, and so on. Each charge must have eight plates. All this must be done carefully enough to avoid contact between the saw cuts of the plates and the electrodes.
The next stage of creating a device for determining water quality is tightening the nuts and insulating the plates, after which we place what we get into a plastic container of a suitable size.
We drill two holes where the bolts touch the walls of the box. It may happen that the bolts do not fit into the container, then cut them and tighten them with nuts to make it tight. Now we drill a hole in the cover for the fittings. To create a tight seal, treat the seam with silicone sealant.
Before you decide to determine water hardness with such a device, you need to check whether it works well enough: connect the device to the power supply, fill it with water up to the bolts, cover it with a lid, connect a tube to the fitting and lower the other end of the tube into water. If a current appears, it can be seen.
Now the current needs to be gradually increased. Distilled water, due to its purity, conducts current very poorly, so to create an electrolyte, you need to add an alkali to it, for example, sodium hydroxide (present in Mole-type pipe cleaners). The safety valve prevents excessive gas accumulation. Congratulations, you have a home-made device that will allow you to determine water hardness!
Read the material on the topic: Filter under the sink: recommendations for choosing
What is total water hardness
Having gained an idea of water hardness and how it affects the body, as well as household appliances and other equipment, we come to the conclusion that it is necessary to normalize it. Homemade methods (boiling or chemical treatment) do not provide adequate water quality. A radical solution to the problem is only possible with the use of modern filtration systems.
Diasel Enginereeng offers highly efficient water treatment systems: softening and ion exchange filters, as well as reverse osmosis. We provide a full range of services for plant design, selection, installation and commissioning of equipment. Our specialists provide their service and warranty service. Go to the "Contacts" section.
Characteristics of hard water
For the human body, hard water with a high calcium content even becomes useful, as it fills it with an element vital for bones. But in addition to this, hard water can provoke the formation of stones in the urinary tract and kidneys, and soft water increases the risk of cardiovascular pathology
When you wash your skin with hard water, it begins to dry out, and when you use soap, it does not lather well. Scale quickly forms on pipes and kettles. But soft water is not as useful as it seems - its use can cause corrosion of pipes and other devices.
For household appliances, high water hardness is often detrimental, especially for washing machines. To prevent breakdowns of the machine or electric kettle, it is necessary to use water filters in everyday life. In the event of a breakdown, repairs will be expensive, so it is better to protect yourself from unnecessary waste in advance.
In highly mineralized water, the concentration of magnesium ions can be several grams, and in salt water - even tens of grams per liter of water.
As for the comparison of groundwater and surface water, the latter has much less hardness, but it is subject to weather changes, increasing as much as possible at the end of winter, and decreasing during floods, when it is diluted with rain and melt water. Maximum hardness is observed in the water of the seas and oceans.