How to determine water hardness
23.03.2018
How to determine water hardness
At first glance, such a concept as water hardness sounds strange - if we were talking about ice, then everything would be much clearer. But in fact, much attention is paid to this characteristic quite often and in different areas, since water affects human health. Below we will answer the question of how exactly and what methods should be used to determine water hardness.
From this article you will learn:
- How to determine water hardness
- How to determine water hardness using instruments
- How to determine water hardness at home
- What salts determine water hardness
Why is it necessary to determine water hardness?
An indicator such as water hardness directly depends on the amount of calcium and magnesium salts it contains. Water hardness is an ambiguous concept, since it can be sulfate or carbonate, this can be determined in different ways.
| Temporary hardness | Carbonate hardness |
| Calcium | Hydrocarbonate |
| Magnesium | Sulfate |
| Strontium | Chloride |
| Iron | Nitrate |
| Manganese | Silicate |
At its core, water hardness is caused by positively charged calcium and magnesium ions that are dissolved in it. This indicator can be determined as follows: if its value is high, then when a container with water is heated, a coating of salts will appear on its walls.
A person is also susceptible to the appearance of a similar plaque, but it manifests itself in a completely different way: stones in the gall bladder, kidneys or liver. It is important to determine the hardness of water, since the possibility of further use of the liquid in a particular area depends on this value.
You should have questions about the hardness of water at home or in the office if scale has appeared on the kettle, the boiler is not working well, or the washing machine is washing poorly. In such situations, it will be useful to determine the value of this indicator, for example, by submitting a sample for analysis to a laboratory.
Read material on the topic: Examination of drinking water
Hardness of natural water
- The hardness of natural water varies widely; it is not the same in different natural waters; in the same body of water its value varies with the seasons. In surface waters, T. reaches its greatest values at the end of winter, and small values during high water. In surface waters, carbonate water usually predominates (70-80% of the total). Magnesium T.V. rarely exceeds 30% of the total, but in some areas (Donbass, Krivoy Rog) it reaches 60% of the total. The temperature of groundwater, especially in artesian wells, changes less throughout the year.
- The hardness of river waters in Ukraine, as well as the mineralization of water, increases from northwest to southeast. In the river waters of Polesie, the hardness is 2-3 mmol/dm³, in the Dnieper - 4-5 mmol/dm³, and in the small and medium-sized rivers of the Azov region - 15-30 mmol/dm³, which limits the possibilities of using local water resources.
- Sea water hardness: Black Sea - calcium 12.0 mmol / dm 3, magnesium 53.5 mmol / dm 3, total 65.5 mmol / dm 3; Caspian Sea - calcium 36.4 mmol/dm3, magnesium 30 mmol/dm3, total 66.4 mmol/dm3; ocean - calcium 22.5 mmol/dm3, magnesium 108 mmol/dm3, total 130.5 mmol/dm3.
How to determine the hardness of different types of water
How to determine carbonate and non-carbonate water hardness
Carbonate hardness is almost 100% of all cases, but in order to determine what it is, you need to use special methods.
What can be done to determine the carbonate hardness of water:
- carry out a chemical test for hardness;
- contact the laboratory;
- buy a special test in the store that will determine the hardness of the water;
- seek help from specialists.
Articles recommended for reading:
- Types of water filters and their characteristics
- How to install a water filter - useful tips
- How to drink water correctly: practical recommendations
How to determine temporary water hardness
The presence of temporary hardness in water is characterized by the presence in its composition of positively charged ions of calcium, magnesium and iron, bicarbonate and hydrocarbonate anions (II). It can be defined as follows: when such water is heated, bicarbonates decompose, but a poorly soluble carbonate precipitate, water and carbon dioxide are formed.
How to determine permanent water hardness
In order to determine the constant hardness of water, it is necessary to identify the content of calcium and magnesium salts in it - sulfates, phosphates and chlorides, which are completely dissolved in water and do not precipitate when boiled. Water can be purified from them using filters with ion exchange resin, reverse osmosis or electrodialysis. To determine the total hardness of water, you need to sum up the indicators of temporary and permanent hardness.
Read material on the topic: How to soften water
How to determine water hardness using special instruments
Water must have an optimal hardness level, since the complete absence of salts is harmful to the human body. If, for example, there are few carbonate salts in the water, this leads to the appearance of cardiovascular diseases.
If we talk about the containers themselves in which water is heated, then soft water promotes corrosion. Therefore, after operating equipment with soft water in thermal power engineering, the surfaces are additionally treated with a solution containing substances that slow down this process.
Thus, water, no matter what source you take it from, has some kind of hardness indicator, and it is ideal if it has an average value, because both an excess of salts and a lack of them lead to certain consequences.
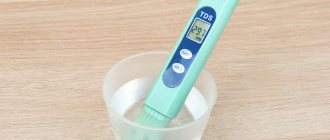
Determining the hardness of water in our time is not difficult - now you can purchase special devices to control this indicator both at home and at work. The best option is the TDS-3 device; it can be found on the market under different trade names.
You can use another device that allows you to determine the hardness of water - an electrolyzer. This is a fairly cheap device.
The Electorlizer will not be able to determine the level of water hardness in numbers, but it will color the liquid a certain color depending on how much salt it contains. After electrolysis, you will also understand which impurities you are dealing with by the color of the water.
You can make such a device yourself; to do this, take:
- stainless steel, size 50 x 50 cm;
- bolts M6 x 150;
- washers;
- nuts;
- transparent tube;
- fittings;
- plastic container with a volume of one and a half liters;
- filter for water purification;
- check valve for water.
The basis for the future device will be a stainless steel sheet, ideally AISI 316L if it is imported, and 03Х16Н15М3 if it is domestic.
Now the stainless steel needs to be marked and cut into 16 identical squares, after which we cut off one corner of each of them, and drill a hole on the opposite corner, which will come in handy a little later. The principle of operation of the electrolyzer is the movement of electricity from a plate of one charge to a plate of the opposite charge, as a result - water breaks down into oxygen and hydrogen.
In order to create a good flow of current, the plates are connected alternately: first a positive charge, then a negative charge, then a positive charge again, etc. The tube is used for insulation; a ring needs to be cut from it, cutting which we get a strip 1 mm thick.
Washers are needed to assemble the plates: screw a washer onto a bolt, then a plate and three washers, then a plate again, and so on. Each charge must have eight plates. All this must be done carefully enough to avoid contact between the saw cuts of the plates and the electrodes.
The next stage of creating a device for determining water quality is tightening the nuts and insulating the plates, after which we place what we get into a plastic container of a suitable size.
We drill two holes where the bolts touch the walls of the box. It may happen that the bolts do not fit into the container, then cut them and tighten them with nuts to make it tight. Now we drill a hole in the cover for the fittings. To create a tight seal, treat the seam with silicone sealant.
Before you decide to determine water hardness with such a device, you need to check whether it works well enough: connect the device to the power supply, fill it with water up to the bolts, cover it with a lid, connect a tube to the fitting and lower the other end of the tube into water. If a current appears, it can be seen.
Now the current needs to be gradually increased. Distilled water, due to its purity, conducts current very poorly, so to create an electrolyte, you need to add an alkali to it, for example, sodium hydroxide (present in Mole-type pipe cleaners). The safety valve prevents excessive gas accumulation. Congratulations, you have a home-made device that will allow you to determine water hardness!
Read the material on the topic: Filter under the sink: recommendations for choosing
How to determine water hardness using soap
This method was described by I. Sheremetev. It is based on the difficulty of washing laundry soap in hard water. Soap foam appears when soap binds excess calcium and magnesium salts.
You can determine the hardness of water using this method in the following way: take 1g of laundry soap, grind it and gradually, carefully, avoiding the appearance of foam, dissolve it in a small amount of hot distilled water.
Pour the resulting solution into a cylindrical glass, now you need to add distilled water to a level of 6 cm if the soap is 60%, or to a level of 7 cm if the soap is 72% (this percentage is indicated on the bar of soap itself). For every centimeter of available solution there is a quantity of soap that can bind hardness salts; they contain 1°dH per 1 liter. water. Now we take a liter jar and fill it halfway with the water whose hardness we want to determine. Then, with constant stirring, gradually pour in the prepared solution. At first, only dark flakes will be visible, and then colored bubbles will appear. If a dense white foam forms, this indicates that all the hardness salts in the water being tested are bound. Now we need to calculate how many centimeters of the prepared solution we poured out - each centimeter bound in half a liter of water the amount of salts corresponding to 2°dH, that is, if 4 cm of soap solution had to be added to obtain foam, then the hardness of the water being tested is 8°dH.
If the solution has already run out, but there is still no foam, we are dealing with water hardness exceeding 12°dH. Then, in order to determine the desired value, we repeat the experiment, but dilute the test water twice with distilled water. The result that we get as a result of repeated analysis should be multiplied by two, this will be the desired indicator of water hardness.
Ordinary glasses, as a rule, have approximately the following parameters: volume - 200–250 ml, height -10 cm, lower diameter - 55 mm or more, upper diameter - 73 mm. Thus, its average diameter is approximately 63 mm. You will be able to most accurately determine the hardness of water if you use a chemical cylindrical beaker with a diameter of about 6 cm. Of course, you can also use a conical beaker, but then the error will be greater. I would like to note that you should not expect absolute measurement accuracy from this method, but an accuracy of 1–2 °dH is quite enough to determine the state of water. This error is largely a consequence of the difference in the upper and lower diameters of the vessel in which the soap solution is located, and it is also influenced by the quality of the soap, distilled water and your experience in performing this analysis.
This method is very simple and allows you to independently determine the hardness of water, so such a small error in determining this indicator should not force you to refuse to use it.
Read material on the topic: Water with lime: content standards and cleaning recommendations
Hardness units:
There is no single unit of measurement for hardness. In Russia, in accordance with Gosstandart, the unit of water hardness is set to mole per cubic meter (mol/m3). In Germany - 2.8DH°; in France - 5F°; in America - 50.05 ppm CaCO3.
Classification of water by hardness:
| Characteristic | Hardness, mEq/l |
| very soft water | up to 1.5 mEq/l |
| soft water | from 1.5 to 4 mEq/l |
| medium hard water | from 4 to 8 mEq/l |
| hard water | from 8 to 12 mEq/l |
| very hard water | more than 12 mEq/l |