The main parameters of water in an aquarium include: total hardness (GH), carbonate or temporary hardness (KH) and pH (pH). These indicators are of key importance when keeping fish and freshwater shrimp and every aquarist should know about them.
First, let's remember the school chemistry course on the dissolution of substances. When a substance is dissolved in water, it dissociates, that is, it breaks up into negative (anion) and positive (cation) ions. For example, if ordinary table salt, sodium chloride (NaCl), is mixed in water, it dissociates the Na+ cation and the Cl- anion.
Dissociation of sodium chloride (NaCl)
Table salt will disintegrate into ions: Na+ cation and Cl- anion.
Solutes behave similarly, on which the GH, KH and indirectly pH indicators directly depend.
pH and carbon dioxide
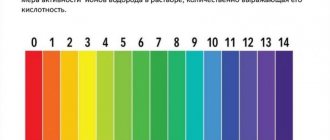
The pH value tells you whether water is acidic or alkaline. It denotes the concentration of hydrogen ions in water and represents its negative decimal logarithm - -log[H+]. Water is considered acidic if the pH is below 7 units, and alkaline if it is above 7. pH values typically range from 0 to 14 units. In aquaculture, the acidity range is 6.5-9.0.
Fish and other vertebrates have blood with a pH value of 7.4. The blood of fish is in close contact with water (the interface is 1-2 cell layers). In the pond, it is recommended to maintain a range close to the pH of the fish's blood - 7.0-8.0. If the pH drops below 5 or rises above 10 (i.e. low alkalinity, coupled with active algae photosynthesis), the fish will feel sick and die.
Pond pH values vary throughout the day. At night, the concentration of dissolved oxygen decreases because photosynthesis stops, plants and animals breathe and use up oxygen. When planted tightly, the concentration of carbon dioxide becomes high as a result of respiration. Free CO2 reacts with water to form carbonic acid (H2CO3) and the pH decreases:
H2O + CO2 = H2CO3 = H+ + HCO3—
The table summarizes the relative changes in dissolved oxygen, CO2 and pH concentrations over a 24-hour period
| Time | Dissolved oxygen | Dissolved carbon dioxide | pH |
| Day | Increasing | Decreasing | Increasing |
| Night | Decreasing | Increasing | Decreasing |
Carbon dioxide rarely has a direct toxic effect on fish. However, its high concentrations lower the pH and, by reducing the pH in the blood of the gills, limit the ability of the fish's blood to carry oxygen. At a given oxygen concentration (eg 2 mg/L), fish may suffocate when CO2 levels are high or remain unharmed when CO2 levels are low. Catfish are tolerant of CO2 concentrations of 20-30 mg/l if the gas accumulates gradually and the oxygen level is 5 mg/l. In a reservoir or natural pond, the CO2 concentration rarely exceeds 5-10 mg/l.
High carbon dioxide concentrations are almost always caused by low dissolved oxygen concentrations (high respiratory activity). To increase low oxygen values, water is aerated. It also helps reduce high CO2 levels due to back diffusion of gas into the atmosphere. Chronically high CO2 concentrations are reduced by adding hydrated lime Ca(OH)2. Approximately 1 mg/l of lime removes 1 mg/l of carbon dioxide. However, this treatment should not be done in water with poor buffering properties (low alkalinity) because the pH will rise to levels lethal to fish. In addition, there is a danger to fish if lime is added to water with excessive levels of ammonium. High pH exacerbates the toxic effects of ammonium.
CO2 in the aquarium
Many people probably wanted to have an aquarium with plants or fish at home, but not everyone understands that its choice must be approached with special care. One of the biggest problems is the supply of CO2 (carbon dioxide). After all, plants consist of 40-50% of it.
Brief summary about CO2:
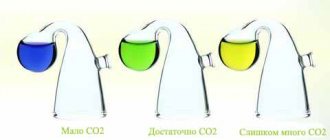
CO2 supply greatly enhances plant growth. The optimal CO2 concentration should be 15−30 mg/l for an aquarium with plants and no more than 30 mg/l with fish. Oxygen is not displaced from water by carbon dioxide. The average CO2 supply value is calculated by the formula: at kHmin = 4 degrees, the supply should be one bubble per minute per ten liters of live volume of the aquarium. The acidity level (pH) should be 6.8−7.2 and this must be carefully monitored, because... Nitrates and CO2 lower the pH level, plus it can change itself throughout the day. It goes down in the morning and goes up in the evening.
To obtain an optimal pH level, it is necessary that the alkalinity measure (kH) of water does not exceed 6 units. To avoid a critical drop in pH, the minimum safe level is kHmin.=4.
The CO2 concentration can be calculated using a formula, but first you need to measure the pH and kH. CO2=3.0xkHx10^(7.00-pH). You can get CO2 from a cylinder or by fermentation.
Plants also need light, but remember that the light intensity and CO2 supply must be directly proportional. The main building block for plant cells is carbon (CO2), so the supply of CO2 is simply necessary for efficient and rapid plant growth. Under normal conditions, plants will grow very slowly or even die, but the supply of CO2 will accelerate the growth rate by 4-6 times! You will be pleasantly surprised by the results when introducing CO2 (carbon dioxide) into the aquarium, just remember to maintain the correct balance with light and liquid fertilizers. Without carbon dioxide, you will only have to watch your plants die. However, CO2 is not the only thing plants need to grow, so immediately after unexpected rapid growth, plants will feel a lack of nutrients. Iron, magnesium, potassium and other microelements are absorbed by plants very quickly and in very large quantities, so the supply of carbon dioxide (CO2) must be coordinated with the supply of liquid fertilizers.
What plants need to grow well. Firstly, good soil with the properties necessary for the plant. Secondly, a constant supply of carbon dioxide (CO2). Thirdly, the plant must have a constant supply of nutrients. Fourthly, there is a sufficient amount of light and the correct composition of the spectrum.
Why do plants need CO2?
Anyone who wants to have an aquarium should understand that all plants are made of C (carbon) and without it they will not survive. Plants eat by performing photosynthesis. This process is not possible without oxygen, carbon, light, etc... Each of the ingredients must be supplied in a certain quantity and duration, otherwise photosynthesis will not occur.
There have been many studies that have shown that given a certain amount of light, CO2 and nutrients, they are the main growth factor. One such study was conducted at Tropica, where Riccia was grown for two weeks. The study showed the following results: If little CO2 and light are supplied, plant growth increases 4 times. Without CO2 supply and little light, growth drops to zero. Low CO2 and large amounts of light increase plant growth 6 times. A lot of CO2 and a lot of light from 1 gram grows 6.9 grams.
Conclusion: If we want a good result, then we should not increase the amount of just one “ingredient” ( [email protected] or light) - this will not give much effect, but with an equal increase the result will amaze you! What if you do what many inexperienced aquarists do, for example, keep the aquarium in the dark without CO2 supply? The plant will only have enough energy to temporarily maintain life.
Why do you need to follow the rules?
You won't have to wait long. To give the composition the desired look. Only 1.5−3 months. You can trim plants more often, edit the composition in more detail, and make it the way you want. Young leaves look better, which means the composition will be better. If you want to aspire to the work of Takashi Amano, then fast plant growth is a must.
Even small doses of carbon dioxide can lead to a 4-fold acceleration of plant growth, and even in dimly lit rooms. This happens because, without any harmful consequences, the plant begins to produce an order of magnitude more chlorophyll, but does not destroy the energy balance. Thus, to extract carbon dioxide (CO2) from water, the plant begins to spend less energy; in fact, the plant spends more energy on optimal processing of the small fraction of light energy given to it. In this way we can very effectively increase the growth of a plant without oversaturating it with light, since it can fully use the light given to it. Because excess light can adversely affect the healthy growth of the plant. But in any case, if you correctly increase the supply of carbon dioxide and light, this will produce a much better effect than improving one thing. You can see how each photon is used in photosynthesis, regardless of the angle at which it hits the leaf, in the graph below. This graph clearly shows the dependence of this process in the use of carbon dioxide molecules on light. So, from all of the above we can draw two conclusions. First: it is very important to balance the supply of carbon dioxide (CO2) with the intensity of lighting and vice versa. Second: even if you supply low lighting, it is recommended to maintain the level of carbon dioxide (CO2) at least 15 mg/l. Although it is better to always maintain the supply level around 30 mg/l. The mistake of many aquarium plant lovers is inexperience and ignorance of the methods of enriching plants with light and carbon dioxide. Usually in such cases, the growth rates of plants in such people are at the level of the yellow line, in rare cases - on the green line. You can achieve the blue line by simply increasing the intensity of the supplied light. But there is a great danger of algae here. Only if you match the supply of carbon dioxide (CO2) with the intensity of the supplied light, can you increase the growth rate significantly, that is, reach the red line. You will be surprised how quickly your plants will grow!
Why CO2?
Plants can consume carbon in two forms: gaseous (CO2) and dissolved in water - bicarbonate (HCO3-). Plants give their preference to pure CO2 - this is due to the fact that for photosynthesis it is necessary to utilize bicarbonate, and this is difficult for plants to do. Therefore, dissolved CO2 is a more profitable way to obtain it.
What should the CO2 concentration be?
I think everyone knows that CO2 is highly soluble, whether in air or water. CO2 dissolves much more slowly in water than in air, but aquatic plants have thought of everything! They have a special layer that speeds up this process; land plants also have it, but it is much thinner than aquatic plants. In aquatic plants, this layer is about 0.5 mm. To ensure optimal photosynthesis for aquatic plants, the CO2 concentration should be 15−30 mg/l, without exceeding the concentration for fish of 30 mg/l. All this is necessary to create a natural environment that creates the main limiting factors for photosynthesis.
CO2 and Oxygen.
Many people are greatly mistaken when they think that oxygen is not displaced from water by carbon dioxide, and that oxygen in large quantities is necessary for fish to breathe. No! This is wrong! In fact, the oxygen level during the day rises to 11 mg/l, which exceeds 100%. This occurs due to active plant growth. The level drops in the morning to 8.0 mg/l under conditions that the water temperature is 24C. For normal life, fish need 5 mg/l (60%) of oxygen dissolved in water.
Should I turn CO2 on or off at night?
There are two opinions on this question. In the first case, they believe that it is possible to do without CO2. Since by morning the oxygen level remains high and the oxygen level remains high and the acidity level is normal, if the aquarium is not more than 1200 liters and does not contain many fish, then you can do without the initial supply of CO2. The second side believes that CO2 should be supplied 1-2 hours BEFORE turning on the lights. Since photosynthesis is most active in the morning, O2 levels are much lower than usual.
Balance of CO2 and light.
As we have already said, the intensity of the light must match the intensity of the CO2 supplied. Even Tropica research confirmed the words of Takashi Amano that if the concentration of supplied light and CO2 is not uniform, then it will only cause harm and not a bit of benefit. Everyone talks about it, but you don't always need a large amount of CO2, we can see this from the photosynthesis formula: 6CO2+12H2O-> C6H12 O6+ 6H2O. At this moment, the plants actively release oxygen, but despite this, the plants become increasingly weaker. Thus, plant consumption of nitrogen and phosphate is reduced. If there is not enough CO2 in the aquarium and there is more than enough light, then algae will begin to appear. No fertilizer should be added. This will cause even more harm. But too much CO2 can become toxic to fish and other aquarium inhabitants. Each plant needs a certain amount of light, and therefore a certain amount of CO2. Some need more light, which means more CO2. Takashi Amano believes that there are no simple or complex plants, there are simply plants that love light and those that love shade. The amount of light and CO2 supplied is the only difference. If you want to start an aquarium, then you should calculate from the very beginning. How much light and CO2 will you supply to your plants so that this does not cause inconvenience in the future?
How much CO2 do you need?
Don't think that's all, you also need to monitor the pH and CO2 balance. To regulate all this, it is necessary that kH, pH and CO2 have the following parameters: kHmin = 4 degrees, pH in the evening = 7.2, and in the morning = 6.8, under such conditions CO2 will acquire parameters from 15-30 mg/l. This needs to be understood by everyone who wants to have or has an aquarium, and understand that all this is interconnected. The more hydroxide ions in water, the lower the pH. The reaction of water can be alkaline (pH>7.0), neutral (pH=7.0) and acidic (pH The concentration of CO2 dissolved in water in nature is much lower than necessary for the underwater kingdom, but in fresh water bodies the opposite is true in relation to it for the inhabitants, the level is too high and is constantly renewed due to the flow and the release of sediments at the bottom. If you do not artificially enrich the water with CO2, then the plants’ own reserves will only be enough to maintain life and, of course, there can be no talk of any growth. You can calculate the flow rate using the following formula, the main thing is that kH = 2-4 with 1 bubble per minute per 10 liters of water: CO2 = 7-19 mg/l at pH = 6.8-7.2 If kH is higher than normal, then you need to calculate it using the formula : kHx V(water) \ 30. Above we already talked about how to properly use large concentrations. But they are designed only for the supply of CO2. Do not forget to monitor the growth of the plant, do not make stupid mistakes, and most importantly, do not forget that the plant needs same amount of light and CO2.
How does CO2 affect acidity levels (pH).
As mentioned above, plants need carbon to grow. It is also recommended to keep the pH level low. By adding CO2 to the aquarium water, we accomplish both tasks. This occurs due to the fact that when CO2 enters water, carbonic acid begins to form. Water combines with CO2 (H2O+CO2=H2CO3). The resulting acid dissociates into ions (H+) and bicarbonate (HCO3-) (the basis of KH). And with an increase in the concentration of hydrogen cations (H+), the pH value decreases. Thus, we simultaneously provide the carbon necessary for plant growth and lower the pH value to a more favorable level. But nevertheless, we increase the level of carbon dioxide (CO2), due to a decrease in pH. (see below in the “pH” section). The concentration of carbon dioxide in water, as well as the carbonate buffer KH, greatly influence the pH value. Because of this, the bond (pHKH dissolved CO2) will be rigid. Now we need to coordinate the supply of carbon dioxide along with what pH level we want in the aquarium. And the pH value is precisely determined by the presence of the carbonate buffer KH. That is, the only thing we can control from our 3 indicators (pH, KH and CO2) is carbon dioxide CO2, since the rest are given values that are optimal for normal plant growth. Thus, now we must adjust the CO2 supply to the optimal pH level, which should be pH = 6.8−7.2, and not just to the desired level of carbon dioxide concentration in the water. For all this we need water with hardness dGH = 4−10, and actually with the initial KH = 2−8. Then the optimal concentration should be CO2 = 15−30 mg/l and pH = 6.8−7.2.
Plants only need pH=6.8−7.2.
Plants want more CO2.
As already mentioned, plants need a lot of CO2, because they themselves consist of 40-50% carbon and it is logical that the best source of energy for them will be carbon dioxide. In water it can be found in two forms: as bicarbonate (HCO3-) and carbon dioxide. They diffusely absorb CO2 through the cell walls, thereby saturating their plant body with nutrients. Many plants have chosen this way of absorbing energy, because it is much easier for them. Since, when absorbing bicarbonate, they must first absorb HCO3− and only then extract CO2 from it and become saturated with it. This happens because bicarbonate contains bound CO2. Now you understand why not many plants choose the second method of absorbing CO2. Many of them are simply not capable of this, and this is understandable, because this is a very complex chemical process.
In soft water with a pH less than 7, 70% of the CO2 will be available and assimilated by plants and only 30% will be in bicarbonate. This means that the lower the acidity of the water, the more oxygen the plants will be able to absorb, since it will be in a form that is easily absorbed by plants (gaseous). Let me explain, this means that in soft water with kH = 2−6, plants will receive much more carbon than in hard water.
Will the pH remain stable with the simultaneous activity of biological substances.
Maintaining a stable pH level in the aquarium. Weak acids may have special chemical properties, the result of these properties is called buffering. The dissociation of weak acids in water forms acid-base pairs that have a logarithmic ratio to each other. When adding acids and bases to water, the pH value will not change much, that is, on a graph of alkalinity/acid versus pH value, we might see that the line below or above a certain pH value will be flat. This state of the pH value is called the “equilibrium point”, when the line is almost flat, that is, no matter how many bases or acids we add, it will not greatly affect the pH level. Moreover, what is also important, there is not one equilibrium point, and can vary depending on the acid. For example, the equilibrium point of carbonic acid (H2CO3), which we get when adding carbon dioxide to water (see above), is pH = 6.37. Due to the natural biological production of nitrates (NO3) in the aquarium, which are acids, the hydrogen level may drop from just above the carbonic acid equilibrium point. And this pH level (pH = 6.37) is almost ideal for aquarium plants, so we need to strive to maintain exactly this pH level. The buffering of the acid will take a long time before the pH level reaches the desired result, this is due to the fact that the initial pH level will be above the equilibrium point, and we need to shift it towards the carbonic acid equilibrium point. This will be for you the secret to the stability of the pH level, (pH = 6.8−7.2), as the best for Nature Aquarium.
Ammonium and toxic ammonia, what should be the ratio between them.
We all know that ammonia (NH3) is one of the forms of ammonium (NH4+) and, moreover, it is very harmful to life, even in small quantities (0.06 mg/l). The ammonium/ammonia ratio depends on the pH amount. If the pH is lower, then there is correspondingly less harmful ammonia in the aquarium. It will be about 0.5% if the pH level is 7, but if the pH is higher, such as 7.5, then the ammonia will be 4%. Which is completely unacceptable! So, you need to remember one simple rule: if the pH (water acidity level) is more than 7.0, then the amount of ammonia increases and harms your plants and fish. The absence of ammonia can be guaranteed in one case if at pH = 6.8 - 7.2 in NA, then the proportion of NH3 = 0.4−0.8%. This is because NA maintains low levels of NH4+\NH3(ammonium\ammonia).
Nitrifying bacteria and their activity.
Bacteria are active at 85% of maximum at an acidity level of 6.6. Bacteria have never worked, and will not work at their maximum. With the slightest change, they can increase or decrease their activity. Even if the water condition worsens, they will cope with the load, slightly increasing the activity of their activities, and will maintain a stable position of the aquarium. The same stability margin will be created as with the pH point. (pH=7.5−7.8 at this parameter maximum nitrophification activity is observed, slows down at pH=7.5).
And so now everyone understands what the pH value should be (6.8−7.2) for good growth and long life of plants. Now let's decide what the kH indicator should be.
1. It is necessary to take into account that water at kH = 2−5 is already acidic, therefore it is automatically buffered at pH = 6.0−7.3. Since it does not contain carbonic acid (H2CO3), but carbon dioxide in large quantities. To avoid the pH dropping lower than is generally possible, the minimum hardness level must be at least = 4.0 while simultaneously supplying CO2.
Why is this particular level needed and why not more? Yes, because if the water is too hard, that is, kH>7.0, then the pH will be equal to = 7.8 and then you will have to exceed the CO2 supply rate permissible for fish. And it should be no more than 30 mg/l. And then there will no longer be any ways or opportunities to reduce the level of acidity in the water even a little. But you can’t underestimate the level of hardness either; I remind you that it’s not lower than two. Then you will have to increase the supply of CO2 or you will have to increase the amount of nitrates, and under all these conditions there may be a threat of a drop, and a sharp level of acidity is less than 6.8. This will be simply terrible for fish and plants.
2. To maintain the stability of acidity in water, it is necessary that the hardness level be at least 4 BEFORE adding CO2, so that the carbonate buffer does not disappear at any moment, which can lead to a decrease in acidity.
I also hope you remember that pH-kH-CO2 are dependent on each other. Therefore, according to Table 1 of the dependence, knowing kH, taking the required pH value, we can find carbon dioxide. That is, what concentration of carbon dioxide will be obtained if we take certain parameters pH and kH.
For example: we observe that with pH = 6.8−7.2; kH= 4−5, then the concentration of carbon dioxide (CO2) will be 7.6− 23.8 mg/l. Using these parameters for water, we obtain a normal amount of pH and CO2. Moreover, there will not be too much CO2; it will optimally saturate the water, which will help accelerate plant growth.
3. In order for plants to freely consume carbon dioxide in large quantities, the water hardness must be equal to 3.5−4 and the water acidity measure must always be less than 7. Based on this (carbonate hardness level), kH plays a major role in increasing the growth of your plants. Unlike general hardness (gH), it does not greatly affect plant growth, so it is a minor, not important factor in the aquarium, but still, in order not to harm the fish, this indicator should not be too high or too low.
Alkalinity
Total alkalinity describes the amount of bases present in water. Typically, carbonates, bicarbonates, hydroxides, phosphates and borates are present in the pond. Carbonates and bicarbonates are the most common and most important components of alkalinity. This is measured by the amount of acid (H+) in water that can be absorbed (buffered) before reaching the designated pH level. Total alkalinity is expressed in mg/L or ppm of calcium carbonate CaCO3. An alkalinity of 20 mg/l is more than enough for good pond productivity. A total alkalinity range of 75-200 mg/L CaCO3 is desired.
Carbonate-bicarbonate alkalinity (and hardness) of surface and well waters is created primarily through the interaction of dissolved CO2 in water and limestone in the soil. Rainwater is naturally acidic because it is saturated with atmospheric carbon dioxide. Once it passes through the soil, each drop becomes saturated with CO2 and the pH drops. Well water is pumped from large, natural underground reservoirs (aquifers) or small, localized areas of groundwater (groundwater). Typically, groundwater has a high concentration of CO2, low pH and low oxygen concentration. The accumulation of CO2 in them is due to the occurrence of bacterial processes in the soil and mineral formations. As soon as rain and groundwater passes through a layer containing calcium limestone CaCO3 or dolomitized limestone CaMg(CO3)2, the minerals dissolve with the formation of calcium and magnesium bicarbonate salts:
CaCO3 + H2O + CO2 = Ca+2 + 2HCO3— CaMg(CO3)2 + 2H2O + 2CO2 = Ca+2 + Mg+2 + 4HCO3—
As a result, alkalinity, acidity and water hardness increase.
Alkalinity, pH and carbon dioxide concentration
In water with moderate to high alkalinity (good buffering capacity) and similar hardness levels, the pH was neutral or slightly basic (7.0 – 8.3) and did not fluctuate widely. Higher concentrations of CO2 (i.e. carbonic acid) or other acids require more bases to lower the pH to neutralize or buffer the acid.
In the table you can see the relationship between alkalinity, pH and CO2 concentration
pH changes over 24 hours in high and low alkalinity water
Factor values for calculating carbon dioxide concentration in water of known pH, temperature and alkalinity (Tucker (1984). Above pH 8.4, CO2 concentration is negligible
The number (factor) noted in the table corresponding to certain pH and temperature values is multiplied by the alkalinity value (mg/l CaCO3). The result of this equation allows you to estimate the CO2 concentration (mg/l).
For example, in a catfish pond pH = 7.2, temperature = 25°C, and total alkalinity = 103 mg/l. Factor value = 0.124. Carbon dioxide concentration = 0.124 x 103 mg/l alkalinity = 12.8 mg/l CO2.
To minimize error in these calculations, pH should be recorded for 30 minutes. Due to several sources of error, direct measurement of carbon dioxide concentrations using chemical tests is preferred.
Making your own CO2 generator
To provide plants with carbon dioxide, you can purchase a CO2 reactor in specialized stores, but such an installation is huge in size and is not cheap. Experienced aquarists prefer to use homemade products - a CO2 generator made independently from scrap materials.
In terms of effectiveness, a homemade unit is not inferior to store-bought analogues, and assembling and installing a CO2 device in an aquarium will not take much time, money and effort of the owner.
Materials for production
To build a CO2 generator with your own hands you will need:
- Transparent plastic bottle with a capacity of 2 l – 1 pc.
- Plastic empty bottle with a wide neck – 1 pc.
- Medical syringe – 1 pc.
- Dropper tube – 1 pc.
- Silicone.
- Back pressure valve – 1 pc.
- Hose – 1 pc.
- Suction cups for fixation.
- Spray.
Assembly
Having prepared the necessary materials, the CO2 system for the aquarium is assembled as follows:
- The piston is removed from the medical syringe and the lower part is cut off, placing a back pressure valve in the syringe.
- Remove partitions and excess protrusions from the bottle cap using a sharp knife. During the operation, be careful not to get hurt.
- The prepared syringe with a valve is connected to the cap using aquarium silicone. A little water is poured into the resulting structure - the device will play the role of a CO2 bubble counter with your own hands.
- The completed counter is connected to a large plastic bottle.
- Holes for the adapter are made in the cap of the second bottle using a thick needle. The adapter from the dropper is inserted into the lid and the hose is connected. The joints are coated with silicone.
- The resulting CO2 generator is filled with liquid. The structures are connected with hoses: from a bottle with a capacity of 2 liters, the tip of the tube is attached to the valve cover, from a needle - to an artificial reservoir.
Compositions for the generator
Having assembled a homemade generator to supply CO2 to the aquarium, you should start preparing a solution that will release carbon dioxide through fermentation. There are many recipes for releasing carbon dioxide, but the most popular and simplest are:
- With soda and food - for preparation you will need 200 g of granulated sugar, a pinch of soda, ½ teaspoon of fish food, yeast and a piece of bread. The ingredients are poured into the bottle, and the mixture is filled with warm water, leaving five cm from the lid. After this, the sprayer of the homemade generator is lowered into the tank and after 10 hours the carbon dioxide supply is checked. If CO2 is not released, then there are leaky places in the structure. An aquarium wash made according to this recipe will release CO2 for two weeks, after which you need to prepare a fresh solution.
- With starch - the aquarist will need 400 g of granulated sugar, 140 g of baking soda, 160 g of starch and 1 liter of water. The ingredients are placed in a saucepan and boiled until thick, then left to cool. The cooled mixture is poured into a fermentation bottle and the sprayer is placed in a pond. Carbon dioxide will be released using this mixture for 3 months.
- With soda and flour - for cooking you need yeast (on the tip of a knife), 100 g of granulated sugar, 25 g of flour and soda. The components are poured into 500 ml of water, mixed thoroughly and poured into a bottle for fermentation. Validity period: 14 days.
- With gelatin - the composition will be valid for 30 days. To prepare, add 30 g of gelatin to 500 ml of water and leave to swell for half an hour. After the specified time has elapsed, add the same amount of water and 1 tbsp to the mixture. spoon of soda. The mixture is placed on low heat until the substances are completely dissolved. After this, the mixture is poured into a fermentation bottle, dry yeast is added and the lid is closed.
- With citric acid - the most popular composition recipe. Citric acid and soda are supplied with CO2 to the aquarium during daylight hours, and it is prepared very simply: you need to add 10 g of citric acid and the same amount of soda, mix and pour into a pre-moistened container. The supply of CO2 to the aquarium with citric acid and soda is ready.
Alkalinity, pH and photosynthesis
Bases that cause alkalinity also neutralize acids. Carbonates and bicarbonates can react with acids and alkalis, and buffer (minimize) pH changes in the environment. The acidity of water with high buffering properties ranges from 6.9-9 units. In water with low buffering properties, the pH can reach alarmingly low values (carbon dioxide and carbonic acid are formed due to respiration) or alarmingly high values (high photosynthetic activity).
Phytoplankton are microscopic or near-microscopic aquatic plants responsible for producing a significant portion of the oxygen in the pond and carrying out photosynthesis. When the pH is stable at about 6.5 or higher, alkalinity improves phytoplankton productivity because it increases the availability of nutrients (soluble phosphates). Alkalinity above 20 mg/L traps CO2 and increases its concentration available for photosynthesis.
Since phytoplankton use CO2 in photosynthesis, the pH in the water increases as the concentration of carbon dioxide and carbonic acid decreases. In addition, phytoplankton and other plants convert bicarbonates (HCO3—) into carbon dioxide for photosynthesis and release carbonates:
2HCO3— + phytoplankton = CO2 (photosynthesis) + CO3-2 + H2O CO3-2 + H2O = HCO3— + OH— (strong base)
High pH values can also cause a decrease in H+ concentration: CO3-2 + H+ = HCO3- or HCO3- + H+ = H2O + CO2
Carbonate released from bicarbonate through plant biomass can cause a significant increase in pH (above 9) during periods of active phytoplankton photosynthesis. This rise in pH is observed in water with low alkalinity (20-50 mg/L) or in water with moderate to high carbonate alkalinity (75-200 mg/L) and hardness less than 25 mg/L. High bicarbonate alkalinity in soft water is caused by sodium and potassium carbonates, which are more water soluble than calcium and magnesium carbonates, which contribute to hardness. If calcium, magnesium and the resulting photosynthetic carbonate are present, when the pH is above 8.3, limestone forms. Ponds with alkalinity below 20 mg/L typically do not experience algae outbreaks and the resulting photosynthesis-induced pH rise.
Carbonate hardness KH
Alkaline earth metals appear in water as part of other substances. The partners of Ca and Mg are usually chlorides (Cl2), sulfates (SO4), carbonates (CO3) and bicarbonates (HCO3). It is the presence of CO3- and HCO3- anions in water that is designated as carbonate hardness. It is also called temporary, since carbonates are easily removed from water by boiling, settling in the form of the well-known scale.
In contrast to temporary hardness, there is a constant, which is due to the presence of remaining Cl2- and SO4- anions in the water. The term “permanent” is used because boiling cannot remove chlorides and sulfates.
Temporary or carbonate water hardness
The content of additional hydrocarbonates HCO3 in water with temporary hardness.
Carbonate hardness is part of the total hardness and, by analogy with it, it is usually measured in German units of measurement dKH and designated as KH (Karbonathärte hängt). The gradation is similar to GH, but the old hardness range (valid until 2007) is more often used, which is usually limited to the range from 0 to 20 dKH.
It is worth noting that carbonate compounds can be associated not only with alkaline earth metals (Ca and Mg), a striking example of this is ordinary baking soda - sodium bicarbonate (NaHCO3). If soda is dissolved in water, it will not increase carbonate and, accordingly, overall hardness. However, existing KH tests for aquarium water will still show an increase. The fact is that they react to all carbonate and bicarbonate compounds, regardless of whether they got into the water along with alkaline earth metals or not.
Why is KH important?
KH is closely interconnected with another important indicator - which is no less important for fish and plants than overall hardness.
Both indicators directly depend on each other. High pH values mean high KH values. Conversely, acidic pH values can only be achieved at low carbonate hardness values (see Table below for details).
In the aquarium, KH has a special role. Carbonate hardness is not only interrelated with pH, it also serves to maintain its stable value. KH acts as a buffer that prevents rapid and significant changes in pH.
Rigidity
Hardness is an important indicator of water when cultivating fish. It is usually present in hydrochemical analysis. Hardness is determined by the concentration of divalent ions - calcium, magnesium and/or iron. It may include a mixture of divalent salts, but calcium and magnesium always predominate.
Traditionally, hardness is measured through chemical titration. The hardness of water samples is expressed in milligrams per liter of calcium carbonate equivalent (mg/L CaCO3). Calcium carbonate hardness is the main indicator of the amount of divalent salts, which does not distinguish between calcium, magnesium and salts of other divalent elements.
Hardness is often confused with alkalinity (total base concentration). The confusion arises because both parameters are measured in mg/l CaCO3 equivalent. If limestone is responsible for both hardness and alkalinity, their concentrations will be similar. However, if in a solution where alkalinity is affected by NaHCO3, the hardness will be low and the alkalinity, on the contrary, will be high. Acidic, groundwater and well waters may have low or high hardness and very low alkalinity (or no alkalinity).
Calcium and magnesium are important for a number of biological processes in the body of fish (formation of bones and scales, blood clotting and other metabolic reactions). Fish are able to absorb calcium and magnesium directly from water or through food.
Calcium is the most important divalent element in culture water. The presence of free (ion) calcium in the water helps reduce the loss of other salts (ie sodium and potassium) from the fish's internal fluids (blood). Sodium and potassium are part of the blood of fish. They are involved in a number of processes, including cardiac activity, innervation and muscle activity. Research has shown that environmental calcium is also required for the re-absorption of lost sodium and potassium salts. In water with low calcium concentrations, significant amounts of sodium and potassium may leak into the water. For the secondary absorption of these elements, body energy is expended. For some fish species (Sciaenops ocellatus, Morone saxatilis), a high calcium hardness value is important for survival.
The recommended range for free calcium in culture water is 25-100 mg/L (65-250 mg/L CaCO3). Channel catfish are tolerant of low calcium concentrations as long as their food contains minimal levels of calcium, but their growth rate is low. Likewise, rainbow trout tolerate low calcium concentrations (10 mg/L) if the pH is above 6.5. For cultivation of Sciaenops ocellatus, Morone saxatilis or crayfish, a free calcium concentration of 40-100 mg/l (100-250 mg/l CaCO3) is desirable, which corresponds to the calcium concentration in the blood of fish (100 mg/l Ca or 250 mg/l CaCO3). The hardness of water sources for these species should be tested.
Low carbonate hardness is a reliable indicator of low calcium content. However, high hardness does not necessarily reflect a high concentration of this element.
Carbonate hardness of 100 mg/l includes 40 mg/l of free calcium (divide CaCO3 by 2.5), if it is due only to the presence of calcium. Similarly, if the carbonate hardness value is 100 mg/L and is represented by free magnesium, the magnesium concentration is 24 mg/L (divide CaCO3 by 4.12). These factors (2.5 and 4.12) are related to the molecular weight of CaCO3 and the differences in the mass of magnesium and calcium atoms. Where hardness is due to the presence of limestone, the CaCO3 value usually reflects a mixture of free calcium and magnesium. But magnesium still predominates in the mixture.
Limestone can be used in agriculture to increase calcium concentration (and carbonate-bicarbonate hardness) in areas with acidic water or soil. However, at a pH of 8.3 or higher, limestone does not dissolve. Soft, alkaline water can be enriched with calcium using gypsum (CaSO4) or CaCl2. Large volumes of treatment can result in significant costs, and it may be more practical to find another source of water.
Total water hardness GH
Water hardness is determined by the sum of alkaline earth metal ions dissolved in water. These are mainly Calcium ions (Ca+ cations) and Magnesium (Mg+ cations). If they are present in large quantities, then the water is hard, if there are few of them, it is soft.
Total water hardness
Content of Mg+, Ca+ ions and SO4-, Cl- anions in water
Historically, in the aquarium hobby, water hardness is designated as GH (Grad Härte) and is expressed in German degrees dGH (or abbreviated dH), or in American ppm (part per million - one part per million). Both units of measurement can be easily converted to each other.
In Europe and fear in the post-Soviet space, German degrees and gradations of rigidity adopted in Germany are widely used. It is in them that the rigidity is expressed in water analysis tests for most popular brands (JBL, API, Tetra, ADA, Sera and others).
Since 2007, in order to adapt to European standards, the following gradation of water hardness has been in effect in Germany: from 0 to 8.4 dGH - soft, from 8.4 to 14 dGH - medium hard, More than 14 dGH - hard.
The optimal GH range for most fish species is between 4 and 16 degrees dGH.
It is worth noting that some manufacturers of aquarium water tests continue to use the old water hardness range (in force until 2007), which is as follows: from 0 to 5 dGH - very soft, from 5 to 10 dGH - soft, from 10 to 20 dGH - medium hardness, from 20 to 30 dGH - hard over 30 dGH - very hard
In this gradation, the optimal range is considered to be between 5 and 20 degrees dGH.
Why is GH important?
In the wild, water parameters differ from region to region. In each specific area, fish have adapted to their own unique habitat. Species living in soft water, when exposed to a high GH environment, will experience increased osmotic pressure, which leads to stress and metabolic disorders. On the other hand, under conditions of deficiency of some alkaline earth metal ions, especially calcium and magnesium, growth problems will be observed, which is especially critical for ornamental shrimp.
Thus, to ensure favorable conditions, it is important to maintain GH values at the level specified in the description of the aquarium fish.
Effect of pH, alkalinity and hardness on ammonium and metal toxicity
Ammonium becomes more toxic as pH increases. High concentrations of the toxic non-ionized form of ammonium (NH3) form in basic water, while NH4+ predominates in acidic water. Because alkalinity increases with pH, ammonium becomes more toxic in water with high total alkalinity. Hardness is not usually associated with ammonium toxicity.
Metals such as copper and zinc are more common in everyday use (swimming pools, plumbing fixtures and CuSO4). They become better soluble in acidic water. The solubility or free ionized form of these metals is toxic to fish. High total alkalinity increases pH and base availability, which promote the formation of less toxic insoluble forms of copper and zinc. High concentrations of calcium and magnesium (hardness) block the effects of copper and zinc at sites of toxic influence. Therefore, these metals are more toxic to fish in soft, acidic water with low total alkalinity.
Ideally, an aquaculture pond should have a pH of 6.5-9.0, moderate to high alkalinity (75-200 mg/L, but not less than 20 mg/L) and a calcium carbonate hardness of 100-200 mg/L CaCO3. Many principles of chemistry are abstract (buffering, carbonate-bicarbonate) and difficult to understand. But a fundamental understanding of the relationship between pH, CO2, alkalinity and hardness is essential for effective aquaculture management. —— www2.ca.uky.edu/wkrec/interactionsphetc.pdf William A. Wurts and Robert M. Durborow. Interactions of pH, Carbon Dioxide, Alkalinity and Hardness in Fish Ponds. Southern Regional Aquaculture Center. SRAC Publication No. 464. 2012.