In nature, everything is in a state of harmony, and if any imbalance occurs, balance comes quickly. But natural restoration of balance does not occur in artificial reservoirs inhabited by aquatic inhabitants. Man has to take on the role of Mother Nature. Otherwise, even the nitrogen necessary for plant growth can destroy all living things with excess nitrates. What NO3 is, how it affects fish and plants, its determination and maintenance within normal limits will be discussed in this article.
What is NO3 in an aquarium
The content of nitrites and nitrates in an aquarium is closely interrelated, since these are links in one chain that accompanies the life activity of the inhabitants of the tank.
Where do nitrates come from in an aquarium?
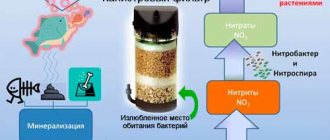
Nitrates accumulate as a result of the nitrogen cycle in the aquarium, a natural process of the life of its inhabitants. At the first stage, the remains of food and fish feces, decomposing, release ammonia into the water. Next, nitrite bacteria (Nitrosomonas) oxidize NH3 to nitrites.
Nitrites are not stable compounds, so they are easily broken down into nitrates, which is facilitated by nitrate bacteria (Nitrobacter). Thus, nitrates are the final link in the nitrogen chain.
Important! Nitrates are also found in tap water. Their level may vary depending on the source and is regulated by relevant regulations and standards. For example, in the UK the permissible level of NO3 in tap water is no more than 5 mg/l.
Nitrogen cycle in an aquarium
Norm and limit values of NO3+
In an established tank, NO3 levels vary between water changes, ideally remaining within acceptable limits. The level of nitrates in an aquarium depends on the sensitivity of its inhabitants to these compounds:
- 15-20 mg/l is the figure to strive for when breeding particularly sensitive species (for example, ornamental goldfish);
- 20-30 mg/l is the norm considered optimal by most aquarists;
- 40 mg/l is the maximum permissible level, exceeding which can cause serious health problems for representatives of aquatic fauna.
With a gradual increase, some types of fish can tolerate even concentrations of up to 400 mg/l without consequences, but most experts are inclined to believe that nitrate levels should be kept as low as possible.
As a rule, the level of sensitivity of a particular fish species to nitrates can be found in tables with data on their care.
Adjusting nitrate levels in the aquarium
Water with a high content of nitrates will look no different and can even please the eye with crystal purity. In this case, the inhabitants of such an artificial reservoir will suffer. The fish will often get sick, cryptocorynes will begin to shed their leaves, and algae will actively grow on the walls of the aquarium and the soil.
To prevent this from happening, it is necessary to regulate the chemical composition of the water, and, if necessary, reduce the level of nitrates or, conversely, increase them. Both the first and second can be done in several ways.
How to reduce nitrates in an aquarium
It is not possible to completely get rid of nitrates in an aquarium. You just need to bring this indicator within the acceptable limits. You can do this in several ways:
- Changing part of the water. A high concentration of nitrates can be easily dealt with by simply replacing ¼ of the total volume of water in the aquarium with fresh tap water. This should be done once every 10-15 days. In this case, it is important to remove remains of organic matter from the bottom, rotted parts of algae and food residues. But this method may not work if the indicator is already exceeded in tap water.
- Reverse osmosis filter for aquarium. This technology for desalinating seawater and purifying drinking water from impurities has been used since the 1970s. Its use will also be justified for filtering all water in an aquarium or tap water prepared for replacing part of the volume in the tank. An additional bonus will be the softening of water, the removal of heavy metal salts and toxic substances from it.
- Denitrifier. A special external nitrate filter uses anaerobic bacteria to reduce nitrates to nitrous oxide, which then simply evaporates from the aquarium. For this process to proceed successfully, it is necessary to ensure a low water flow and proper feeding of the bacteria with lactose or cornstarch. The disadvantage of this method is the high cost of the filter itself and the complexity of operation.
- Filter media. Instead of expensive dinitrifiers, aquarists, in order to reduce the level of nitrates, prefer to use component mixtures (granules or sponges with special impregnation) in the filtration system or separate canister filters. In modern aquarium filters, nitrate reduction can occur by absorbing nitrates or nitrogenous compounds before they are converted to nitrates.
- Biological products. These special chemicals can normalize the concentration of nitrates in sea or fresh water for a long time (up to 1 year), increasing the time between water changes. Additives will help to urgently reduce the content of nitrates and nitrites, chlorine and heavy metal salts.
Changing your aquarium water helps reduce nitrate levels.
Attention! Among the folk remedies that reduce the concentration of nitrates, vodka is often mentioned, but it can only be used in aquariums with sea water, carefully calculating the dose so as not to harm the fish.
If none of the proposed methods could correct the situation, then the aquarist should reduce the number of inhabitants of the tank, reconsider their feeding and plant more fast-growing algae.
How to raise nitrates in an aquarium
Nitrates in an aquarium are not always evil. For example, for tanks that are heavily planted with plants (herbal plants), it may be necessary to increase the level of NO3 compounds, an important element in the nutrition of aquatic flora.
Insufficient nitrogen levels are indicated by slow plant growth, yellowing of leaves that begins at the edges and tips, black areas on fern leaves, and intensive dying of old leaves.
There are two ways to raise nitrates in an aquarium: natural and artificial. In the first case, it is necessary to populate the aquarium with a large number of different living creatures, in particular viviparous fish. They will produce more feces, which, once decomposed, will turn into nitrates, naturally increasing their levels.
Viviparous fish increase nitrate levels
You can quickly artificially increase the nitrate content in water if you add a special nitrogen fertilizer or macrofertilizer to the soil, which, in addition to nitrogen, should also contain potassium and phosphorus.
The following can be used as a top dressing that will increase the nitrate content in aquarium water:
- KNO3 - potassium nitrate or potassium nitrate - this is the cheapest and most accessible option that does not cause excessive algae growth;
- NH4NO3 - ammonium nitrate or ammonium nitrate - is not the best remedy, since it is an activator of algae growth, it is better to use it when water hardness pH> 6.5 in an aquarium without fish or with a small number of them;
- NH2 - amide nitrogen is another good source of nitrates that does not promote algae growth.
For an experienced aquarist, if you have accurate jewelry scales, it will not be difficult to prepare your own nitrogen supplement by mixing urea, ammonium nitrate and potassium nitrate in proportions of 3:5:10 mg/l.
redfield ratio / Redfield proportion
This information is fundamental to understanding the balance of the aquarium's ecosystem and maintaining an almost algae-free environment. For “land” plants, the phosphorus:nitrogen ratio is important only in the sense of ensuring optimal growth, but for an aquarium, the influence of this proportion on the presence of algae as such or certain types of algae is no less important.
“The Redfield proportion considers the optimal ratio of Carbon and Phosphorus necessary for Life. Since the energy requirements of terrestrial and aquatic plants are the same, the optimal C:P ratio is 106C:1P for both. Thus, the total Redfield Proportion (optimal ratio of C to N to P) for terrestrial and aquatic life: on land - 106C:16N:1P; in water - 106C:13N:1P. (approx. transl.: atomic!) We already know that the need for N on land is greater because they need more proteins to create a rigid structure for their body. The downside to this is that since N requirements are lower in aquatic systems, the relative P requirements are higher because phosphorus is evenly distributed between aquatic and terrestrial life. Thus, in water bodies it is usually phosphorus that limits growth.” (Not phosphorus cycle)
Studies have shown that algae growth occurs when there is an imbalance in the phosphorus:nitrogen ratio in the reservoir. This proportion is called the Redfield ratio (RR-ratio). In 1934, American scientist Alfred C. Redfield (1890-1983) discovered that the atomic ratio of CNP in zooplankton in all oceans was 106C:16N:1P. Deviations were no more than 20%. This ratio was also studied to determine the influence of these two elements on the appearance of certain types of algae in water bodies (see Levich/Bulgakov English, Russian) and it was found that a shift in the proportion in one direction or another is characteristic of the dominance of certain species. Green algae exist when there is a relatively large amount of nitrates (NO3>5mg/l) in relation to phosphates in the water*, and conversely, a small amount or absence of nitrogen and a lot of phosphates (PO4>0.15mg/l) leads to the appearance of blue-green algae ( Cyanobacteria). [not to be confused conc. in water with a dosage per week! ]
“The Redfield Ratio considers the optimal ratio of Carbon and Phosphorus necessary for Life. Since the energy requirements of terrestrial and aquatic plants are the same, the optimal C:P ratio is 106C:1P for both. Thus, the total Redfield Proportion (optimal ratio of C to N to P) for terrestrial and aquatic life: on land - 106C:16N:1P; in water - 106C:13N:1P (atomic). We already know that the need for N on land is greater because they need more proteins to create a rigid structure for their body. The downside to this is that since N requirements are lower in aquatic systems, the relative P requirements are higher because phosphorus is evenly distributed between aquatic and terrestrial life. Thus, in water bodies it is usually phosphorus P that limits growth.” (Not phosphorus cycle)
In addition to the Redfield ratio common to a reservoir (see below), each living organism or colony has its own. For example, aquatic plants contain P:N ~1:8-10 (Garten 1976), and algae ~1:14 (Redfield 1958). Conversion to PO4:NO3 by mass will give ~1:5.3-6.7 for plants, and ~1:9.3 for algae. This can be used to determine which element will become deficient first at a certain proportion of PO4:NO3 in fertilizer/water/soil: you need to divide the concentration of the substance by its share in the Redfield proportion. For which element the resulting number is smaller, it will become limiting ( ). For example, if you add a solution with an atomic Redfield ratio P:N=1:7.5 as in the Estimative Index (with PO4:NO3 by weight 1:5), we will conventionally assume that we add PO4 at 1 mg/l with NO3=5 mg/l, which will give by weight pure P~0.33 mg/l and N~1.15 mg/l. We get 0.33/1=0.33 and 1.15/10 = 0.115, that is, in this case, nitrogen N will run out faster. If we add a solution of PO4:NO3 1:19 according to Tropica with an atomic ratio of 1:28.5 and a dose of P=0.33 mg/l and N =4.37 mg/l we get: 0.33/1=0.33, 4.37/10=0.437), that is, phosphorus will run out a little earlier, which gives huge advantages in cases of plant CO2 limitation. With a fertilizer ratio of PO4:NO3=1:15, nitrogen and phosphorus run out simultaneously.
Let's compare three systems using plant composition data (atomic P:N=1:8-10): General practice and Seachem with PO4:NO3=1:15 -> atomic 1: 22.5 –> 2.25…2.8 (phosphorus P will run out faster) Tropica with PO4:NO3 1:19 = atomic 1:28.5 -> 2.85…3.56 (phosphorus P will run out faster) Estimative Index with PO4:NO3=1:5 -> atomic 1:7.5 –> 1:0.75…0.93 (nitrogen will run out faster N). That is, the emphasis is on ensuring that nitrogen N is ALWAYS in excess relative to phosphorus P! ADA liquid fertilizers are specific - since the main source of nitrogen is the Aqua Soi substrate, almost only PO4 is added to the water. Portions of PO4:NO3~1:1.695 for Lights, and 1:1.915 for Shade.
Redfield ratio is often misused by aquarists. To use this ratio correctly for fertilizer formulation, you must first convert the atomic Redfield ratio into a P:N ratio by mass, and then into a mass ratio of PO4:NO3. The conversion factor mass PO4:NO3->atomic P:N is roughly assumed to be 1.5. Atomic Redfield ratio 106C:16N:1P. Converting to the ratio by mass will give 41C:7.2N:1P, converting to PO4:NO3 by mass will give 1:10.4 . The permissible range of atomic Redfield Ratio is 1:15-30 (hereinafter referred to as RRatomic), PO4:NO3 by mass is 1:~10-20.
The best atomic Redfield ratio with maximum plant growth and minimum algae, judging by the graph of Adriaan Briene, is 1:24, which corresponds to PO4:NO3 = 1:16.
Conversion of atomic Redfield Ratio to PO4:NO3 ratio by mass: RRatomic = (NO3/PO4) x 1.5.
Note: Nitrogen in an aquarium is also contained in ammonium NH4+ and nitrite NO2, but their concentration in a healthy aquarium is so low that this can be neglected.
There are also several articles on the Internet incorrectly converting atomic RR to PO4:NO3 ratio by mass. Instead of the formula RRatomic = (NO3/PO4) x 1.5, where RRatomic is the atomic Redfield Ratio and NO3 and PO4 concentration by mass, an erroneous formula was used with a multiplier of 0.7 instead of 1.5.
The correct calculations, Calculator, Table and translation of the atomic Redfield Ratio into the PO4:NO4 ratio by mass are in the article by Adriaan Briene: De Redfield Ratio, de basics part 1, part 2. (Dutch)
C. Baddendorf gives an example of a stable proportion of PO4 = 0.2 and NO3 = 5 mg/l in a beautiful competition champion aquarium in Holland, which was maintained for years. This corresponds to RRatomic~1:37.5, that is, PO4:NO3=1:25 gives a good result because improves Rubisco activity = CO2 consumption (PJAN).
| Determination of the atomic Redfiled ratio by the NO3:PO4 ratio by mass. | |||||||||||||||
| Phosphate [PO4], mg/liter | Nitrate [NO3], mg/liter | ||||||||||||||
| 1 | 1.5 | 2 | 2.5 | 3 | 5 | 7 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| 0.05 | 30 | 45 | 60 | 75 | |||||||||||
| 0.1 | 15 | 22.5 | 30 | 37.5 | 45 | 75 | |||||||||
| 0.175 | 8.6 | 13 | 17 | 21 | 26 | 43 | 60 | 77 | |||||||
| 0.2 | 7.5 | 11.2 | 15 | 19 | 22.5 | 37.5 | 52.5 | 67.5 | |||||||
| 0.25 | 6 | 9 | 12 | 15 | 18 | 30 | 42 | 54 | |||||||
| 0.3 | 5 | 7.5 | 10 | 12.5 | 15 | 25 | 35 | 45 | 60 | ||||||
| 0.5 | 6 | 7.5 | 9 | 15 | 21 | 27 | 36 | 45 | |||||||
| 0.6 | 5 | 6.2 | 7.5 | 12.5 | 17.5 | 22.5 | 30 | 37.5 | 45 | ||||||
| 0.8 | 5.6 | 9.4 | 13 | 16.9 | 22.5 | 28 | 33.8 | 39 | 45 | ||||||
| 1.0 | 4.5 | 7.5 | 10.5 | 13.5 | 18 | 22.5 | 27 | 31.5 | 36 | 40.5 | 45 | ||||
| 1.2 | 6.25 | 8.8 | 11.2 | 15 | 18.8 | 22.5 | 26 | 30 | 33.8 | 37.5 | |||||
| 1.4 | 5.4 | 7.5 | 9.6 | 13 | 16 | 19 | 22.5 | 26 | 29 | 32 | |||||
| 1.6 | 4.7 | 6.6 | 8.4 | 11.3 | 14 | 16.9 | 19.7 | 22.5 | 25 | 28 | |||||
| 1.8 | 5.8 | 7.5 | 10 | 12.5 | 15 | 17.5 | 20 | 22.5 | 25 | ||||||
| 2.0 | 5.3 | 6.8 | 9 | 11.3 | 13.5 | 15.8 | 18 | 20.3 | 22.5 | ||||||
| 2.5 | 5.4 | 7.2 | 9 | 10.8 | 12.6 | 14.4 | 16.2 | 18 | |||||||
| 3.0 | 6 | 7.5 | 9 | 10.5 | 12 | 13.5 | 15 | ||||||||
| – minimal chance of algae growth | |||||||||||||||
| – possible growth of blue-green algae | |||||||||||||||
| – possible growth of green algae | |||||||||||||||
| Optimal Redfiled Ratio (atomic) | 20-24 | ||||||||||||||
| Optimal Range Redfield Ratio (Atomic) | 15-30 | ||||||||||||||
| minimum limit (blue-green algae) | <15 | ||||||||||||||
| maximum limit (green algae) | >30 | ||||||||||||||
How to use this in practice? You just need to make a phosphate:nitrate solution with the correct proportion ~1:10-15 (RRatomic=15-22.5), and in case of temporary imbalance, regular water changes will bring everything back to normal. For the stepwise method of illumination of phosphate, less is needed - PO4:NO3 = 1:15-25 (RRatomic = 22.5-37.5. Deviation from these parameters towards nitrate (RRatomic>1:45) can contribute to the rapid growth of green algae, and towards phosphate (RRatomic<1:10) - blue-green... Nitrogen deficiency is much worse than phosphorus, since it sharply reduces the ability of plants to consume CO2, and their correct proportion is the Main rule for applying N and P. It is this proportion that is used when applying nitrogen and phosphorus with Seachem Flourish Nitrogen™ and Flourish Phosphorus™ products.
PO4:NO3 ratios (by mass) used in aquatic plant fertilizers: Tropica PLANT NUTRITION+ liquid by mass PO4:NO3~1:19 (1.34 N-0.1 P-1.03 K), which is equal to RRatomic~1:28.5 Seachem Flourish Nitrogen™ and Flourish Phosphorus™ ¬ PO4:NO3 by mass ~1:16, which is equal to RRatomic~1:24 PPS-pro by mass PO4:NO3~1:10, which is equal to RRatomic~1:15 Estimative Index by mass PO4:NO3~ 1:5, which is equal to RRatomic~1:7.5 PJAN (PJ Magnin) in the stepwise illumination method (i.e. according to ADA) PO4:NO3 by mass ~1:15-25 (RRatomic~1:22.5-37.5) (intentional limiting plant growth to improve the stability of the absence of algae and maintaining the composition) As you can see, PO4:NO3 = 1:10-15, widely used in our hobby, fully corresponds to the optimal atomic Redfield Ratio 1:15-22.5.
In Takashi Amano's aquariums, the level of phosphates is no more than 0.05-0.1 mg/l, and nitrates are no more than 1-3 mg/l. This does not mean that there may be a lack of nitrogen - the Aqua Soil substrate works so well that the plants never experience a lack of N and almost only PO4 is added to the water (in liquid fertilizers ADA PO4:NO3~1:1.695 for Green Brighty Special Lights and 1:1.915 for Green Brighty Special Shade), that is, ADA uses the ratio PO4:NO3 = 1 to infinity and the plants themselves take as much nitrogen as they need. We can suggest the ideal proportion for an aquarium with plants according to the ADA system: PO4 = 0.1-0.2 mg/l with NO3 = 1.5-3.0 mg/l with PO4:NO3 = 15, which will give an atomic Redfield Ratio ~1:22.5. For systems with fertilization only in water - the same proportion of fertilizers, only the dosage is at a higher concentration - 1.5-3 mg/l PO4 per week. Higher concentrations of phosphates, 0.2 mg/l, are also possible since a high level of phosphate (as well as nitrate) in water itself is not a direct cause of algae. For example, according to the Estimative Index system) you can dose 3 mg/l per week without algae at all. In the ADA system, the dosage in water is much less since the plants receive most of their nutrition through the rich substrate. The difference between them is stability - EI with PO4:NO3 1:5 is less stable because more susceptible to insufficient fertilizer dosage and fluctuations in CO2 concentration.
In a stable aquarium with a normal dosage that does not limit (!) plant growth, the Redfield proportion does not play a special role and PO4:NO3 = 1:5 will not be a direct cause of the appearance of algae (see - permalink). But for now, the dosage is sufficient and stable, but what if there is a lack of nutrition? I believe that when plants are starved of nutrition from too little/irregular fertilization, lack of CO2, or large water changes during an algae outbreak, a PO4:NO3 ratio of 1:10-15 will provide the benefit of less algae growth. unstable aquarium and gives the aquarist more time to correct the error - the system will be more stable (more details). This is far from a panacea for all ills, but the use of fertilizers with PO4:NO3 1:15 can be safely called a “good habit” that will never do any harm - only benefit. This proportion has been used for a very long time. The ADA system has the highest stability and user friendliness with the majority of nutrition in the substrate, the minimum concentration of nutrients in the water, and a special lighting mode (more details). Sometimes you see claims that the ratio of 1:10-15 causes green spot algae, but this problem is as easy to solve as the lack of nitrogen in the EI system - just temporarily increase the dosage of fertilizers or add a couple of doses of KH2PO4 without the slightest danger of causing algae growth.
Note: Such low concentrations of phosphates and nitrates as Takashi Amano does not mean that he introduces them in such small quantities. This means that after applying fertilizers, thanks to optimal lighting and supply of CO2, plants almost completely consume/store them within a day or two, and the concentration of these substances in the water remains extremely low, reducing the growth rate of algae in case of imbalance (in itself, a higher concentration of PO4, NO3, Fe, etc. are not a direct cause of algae growth!). In addition, in the ADA system, the main supply of nutrition is in the substrate, which allows, without lack of nutrition for plants, to limit the concentration in water to the maximum. When the substrate is poor, PO4:NO3 is added more - by 1.5-3 mg/l PO4 per week. Note: The dosage and the NO3:PO4 proportion itself have a significant impact on the aquarium lighting method. For more information about this, see the section Stepped lighting method.
Redfield Ratio does not cancel other basic rules for preventing algae: - optimal plant growth and high biomass due to optimal lighting intensity and corresponding CO2 supply, the introduction of a sufficient amount of PO4:NO3, microelements and compliance with the correct duration of lighting of the aquarium. No Redfield Ratio will help if the plants do not grow well enough (possibly slowly) and their biomass is insufficient - algae will still be present in the aquarium regardless of the proportion, and primarily because there will be a lot of ammonium NH4+. ^
Trophic State.
In addition to the Redfield ratio, there is a method for determining the health of natural bodies of water using the so-called Trophic State. In this case, the Phosphorus:Nitrogen ratio is also used. If a body of water has a low amount of nutrients, then it is said to have a low Trophic level - that is, it is Oligotrophic (literally, “small nutrient base”). If there are enough nutrients but not excessive - Mesotrophic (average amount), and reservoirs with excess food are called Eutrophic (too much). A nitrogen to phosphorus ratio of 10:1 is thought to reduce algae growth, and adding nitrogen will stimulate algae growth; a ratio between 10:1 and 15:1 is considered transitional; and if the proportion is more than 15:1, it is considered that there is not enough phosphorus in the reservoir, and at this and higher ratios, an increase in the proportion of phosphorus enhances the growth of algae. In principle, this corresponds to what was said above. Phosphorus is considered the main limiting factor for the growth of green algae, while at the same time a very low concentration of nitrogen in water does not guarantee the same inhibition of algae growth as a lack of phosphorus. Blue-green algae infestations come from very little nitrogen in the pond, and higher concentrations of nitrates are even better - other types of algae will suppress the blue-green, which will significantly improve the condition of the pond. Note: please note that Total Phosphorus is indicated, and not the concentration of PO4 in the water!
Oligotrophic Total phosphorus (Total Phosphorus) 0.005-0.1 mg/l. NO3<0.3 mg/l. - The body of water is generally clean, deep and free of plants and algae growth. Although beautiful, it contains few nutrients to support large fish populations. However, Oligotrophic waters often develop a good food chain capable of supporting a desirable population for fishery for large game fish.
Eutrophic Total Phosphorus 0.03-0.1 mg/l. NO3=0.5-1.5 mg/l. - the reservoir has a high level of phosphates, a lot of nutrients, a large amount of bottom sediment, and it can support a large biomass (many animals and plants). They usually grow algae or often have algae infestations. There are a lot of fish in them, but they are susceptible to oxygen deficiency. Small, shallow eutrophic water bodies are particularly susceptible to winter blight, which can reduce the number and diversity of fish species. They are often home to hardy fish. Deprived of oxygen in late summer, the hypolimnions of deeper Eutophic reservoirs limit the number of cold-water fish and cause phosphorus to be released from the sediment. Nature Aquarium seems to be a Eutrophic pond.
Mesotrophic Total phosphorus (Total Phosphorus) 0.01-0.3 mg/l. NO3=0.3-0.5 mg/l - occupies an intermediate position between the Oligotrophic and Eutrophic stages.
Hypereutrophic Total Phosphorus >0.1 mg/l. NO3>1.5 mg/l. - too much nutrients leads to complete algae overgrowth.
How to test aquarium water at home
Fish diseases often become a reaction of animals to the chemical composition of water, so the solution to most problems with the flora or fauna of artificial reservoirs begins with water analysis.
Aquarium water contains a large number of different substances in varying concentrations. This makes it impossible to clearly assess whether water is “bad” or “good”, since various indicators need to be analyzed. Among the main ones are:
- hardness (dGH);
- acidity (pH);
- the presence of nitrogen compounds (nitrogen, nitrites, nitrates).
At home, you can analyze all these indicators using special tests (reagent kits with reference cards in the form of colored stripes). This can be a test for one specific indicator or for several.
Set of reagents with a standard scale for testing water quality
This method of analysis does not always give an accurate result. An error may arise at the time of comparison with samples, when it can be difficult to assign a sample to one color or another, but such errors are not critical for home use.
In addition to drop tests, there are test strips that work on the principle of litmus paper. These are budget indicators that can be purchased individually. To analyze, you just need to dip the strip in water and then compare the result with the standard scale. Like droplet tests, they provide an approximate result, which is a disadvantage.
The most accurate assessment of the chemical composition of aquarium water can be made using digital instruments. They have a probe that is lowered into the water, after which specific digital indicators are reflected on the display. But these devices have one significant drawback - high cost.
What does an imbalance of microelements lead to?
Microelements (copper, iron, boron) are just as important as macroelements, although aquarium inhabitants need them in smaller quantities. If you remove even one of them, the consequences will be disastrous for both flora and fauna.
Iron
Iron is an important trace element that plays a significant role in the physiology of aquatic plants. In terms of consumption, it can be placed on a par with such macroelements as nitrogen and phosphorus.
In human blood, iron, as a component of hemoglobin, is responsible for the delivery of oxygen to tissues. Plants need this element for the synthesis of chlorophyll. A deficiency of this substance can lead to a disease such as chlorosis, in which the leaves turn white, but the veins remain green, and over time the shoots die.
Lack of iron leads to chlorosis of aquarium plants
Excess iron is also dangerous because it can provoke the growth of unwanted algae, so its levels must be carefully monitored and adjusted if necessary.
Note! Beginning aquarists often think that tap water ("rusty") or a couple of nails in the aquarium will help maintain the balance of this substance, but this is not the case. In order for iron to be absorbed, it must be in aqueous divalent form, which quickly transforms into trivalent form and precipitates.
Instead of rusty nails, you can prepare your own fertilizer from 500 ml of distilled water, 20 g of citric acid and 20 g of iron sulfate. The resulting yellow solution will contain iron in an amount of 8 g/l and can be used for 30 days without loss of properties.
Bor
Although boron is needed by plants in an aquarium in small quantities, its deficiency or excess can seriously affect the appearance and health of aquatic flora. If the boron concentration is significantly reduced, then plants react to this by deforming the leaves and reducing their size, darkening and death of growing points, and in serious cases, the root system dies.
Correcting the situation is much more accessible and easier than with hardware. It is enough to purchase boric acid or borax at the pharmacy. It will need to be applied within 10-15 days depending on the state of the vegetation at the rate of 10-20 mg per 100 liters of water.
Boric acid allows you to normalize the level of boron in water
Plants will not be happy with excess boron. In this case, their leaves will turn yellow at the edges, take on a dome-like shape, and then fall off.
Does the nitrate to phosphate ratio affect algae growth in an aquarium?
It is believed that the appearance of certain algae is associated precisely with a deviation from the Redfield ratio. You can read on some sites that if the deviation is towards nitrates
- then green algae appear.
If the deviation is towards phosphates
, blue-green algae will appear.
If the Redfield ratio is followed, then you will have a clean aquarium with plants and no algae. But there is an obvious contradiction here. Different unwanted algae require different ratios of nitrate and phosphate
.
Some algae need more nitrate
, some
phosphates
.
But for all types of aquarium plants, supposedly one single ratio of nitrate and phosphate
.
Where is the logic? But there is no logic. In fact, different types of plants also need different ratios of nitrate and phosphate
, just like algae.
To confirm this, we conducted a series of analyzes of different plants from aquariums. The first example is that the Japanese Blix, beloved by many aquarists, has a nitrate to phosphate ratio of 5/1 . And Redfield's ratio is 10/1. How do you like this deviation? Twice. Now imagine that your aquarium is almost entirely planted with this beautiful plant. And so you read that on average all plants consume nitrates and phosphates
in a ratio of 10/1 and start fertilizing with this ratio. What will happen? Blixa will lack phosphates. It will become smaller and grow slowly.
Another equally interesting example. The most popular plant for creating a green rug is Hemianthus cuba. We also analyzed it. Nitrate to Phosphate Ratio
it's as much as
30/1 .
The deviation from the Redfield ratio is three times, and in the other direction. See what a huge difference it is. 5/1 for Blix, 10/1 for Redfield and 30/1 for Hemianthus Cuba. What fertilizer is needed for an aquarium in which only Hemianthus cuba grows? Obviously, it should not contain a lot of phosphates as is the case with Blixa. And even using fertilizer with a ratio of nitrates and phosphates according to Redfield, a lot of excess phosphates will remain in such an aquarium, which algae will happily take advantage of. Often this is a green coating on the stones, because such aquariums are usually designed in the Iwagumi style. If we take long-stemmed species, there is also a wide range of preferences among them. Ludwigia brevipes likes phosphates more. It consumes nitrates and phosphates in a ratio of 7/1 . But Didiplis prefers nitrates. It has a nitrate to phosphate ratio of 16/1 . Mosses. Using the example of Fissidens, it is clear that he just likes Redfield’s ratio. It has a very close nitrate to phosphate ratio of 11/1 . Cryptocoryne beckett, much like Cuba, needs only a small amount of phosphate. She has a ratio of 23/1 .
We analyzed a whole list of plants. They can differ greatly in their preferences from each other. Some plants like nitrates more, others prefer phosphates. The difference can be significant. But the average ratio of nitrates to phosphates is very close to the Redfield ratio. According to our list, it's 14/1 . What does this affect? To illustrate, I want to give one example from life.
Using special aquarium chemicals
It is not always possible to create a favorable habitat for fish and plants in an aquarium in a natural way, but do not be upset. Modern aquarium chemistry will help solve certain problems.
All special chemicals for aquariums can be divided into the following groups:
- Fertilizers for plants that will provide the green inhabitants of the tank with all the necessary micro- and macroelements. They are produced in several forms. Liquid fertilizers are dissolved in water, solid granular fertilizers are mixed with soil.
- Water conditioners are preparations that will prepare tap water for an aquarium, removing chlorine from it and binding heavy metals so that they do not harm fish and plants.
- Fish color stimulants - the chemical composition of water can significantly change, and not for the better, the color of brightly colored fish. Special chemicals containing the necessary biological additives to preserve color will help correct the situation.
- Preparations for the control of algae that do not have a negative effect on fish and plants. Algae do not provide any benefit, but only upset the balance, taking away useful substances, but they can simply be defeated with the help of modern aquarium chemistry.
- Remedies for water cloudiness. The water in the aquarium becomes cloudy after replacement or from small particles of uneaten food by the fish. In the first case, the situation returns to normal after a few hours, and in the second, the use of special drugs is necessary.
- Preparations that stabilize and maintain the environment at the proper level. It is not always possible to normalize the hardness and acidity of water, or to balance the content of phosphates and nitrates, but aquarists will be able to cope with these tasks using drugs from this group.
Remedy for water cloudiness
Drugs for the treatment of various diseases in fish are used directly in the aquarium, acting strictly according to the instructions, in order not only to cure sick individuals, but also to protect healthy ones from infection.
What does the nitrate to phosphate ratio really affect? Experiment.
This incident, which occurred in 2012, prompted us to conduct a study of plant compositions. At that time we were reorganizing our experimental nursery of aquarium plants. And in order to maintain order, they decided to keep only one or two types of plants in each aquarium. In one aquarium there is Blix, in the second there is Lyaliopsis, in the third there is Hydrocotylus, in the fourth there is another species and so on. And of course, we used our AQUAYER Udo Ermolaev MACRO+ and MICRO+ fertilizer in all of them. One set of fertilizers for everyone. And what do you think? Many plants did not grow well. How so? This is our fertilizer, which we tested on a lot of different aquariums and it never failed. And here's the problem. Why?
We started to figure it out. What have we done? We took all these species and mixed them back together so that there were not one or two species in one aquarium, but several species. The bigger, the better. Nothing else was changed. The same fertilizers, the same lighting, soil, everything is the same. They just made a wide variety of species in one aquarium. And everything became wonderful. All the plants grew and looked great, even those that had never grown alone before.
Why did it happen? Because the 10/1 ratio works when there are several or many different types of plants in the aquarium. When there are many different ones in one aquarium, they compensate each other. Blixa and Laliopsis took a lot of phosphate and left a lot of nitrate in the water, and this excess nitrate was taken by Cubaa with Cryptocoryne. But when there are only one or two types of plants in the aquarium, there is no one to compensate and you need to select the ratio of nitrate and phosphate for these plants. And it could be 5/1 or 20/1.
Where to buy the necessary substances
All necessary substances for caring for the aquarium and its inhabitants can be purchased at specialized pet stores. If there is none nearby or the assortment is poor, the necessary goods can be found in online stores. This method of making a purchase allows you to leisurely familiarize yourself with the description of the drug, as well as get a telephone consultation about the properties of interest from the seller.
The presence of ammonia, nitrites and nitrates is inevitable in an aquarium, so you need to learn to carefully monitor the concentration of these substances in order to lower or increase their level in time. A careful approach to water quality will help maintain the health of the inhabitants of the reservoir in order to fully enjoy the beauty of the underwater world.
Did you learn something new? Share about it in the comments!
Need a little more phosphates
Another very important point. During our observations, it turned out that in an aquarium, a little more phosphates are needed than the plants require. For what? Who else needs phosphate in an aquarium besides plants? The fact is that phosphates can bind to precipitated iron. It is known that iron is very unstable in aquarium conditions and quickly precipitates in the sludge even if it is introduced with very strong chelators. That’s why they pour a lot of iron into plants. By the way, we also analyzed plants for iron content. So, the average nitrate/iron ratio in plants according to the mentioned list is 650/1! And the nitrate/iron ratio in fertilizer systems of various manufacturers ranges from 100/1 to 20/1. The excess iron in fertilizers compared to how much iron reaches plant cells is simply enormous. And all this excess falls into the sludge. Just don’t think that if you pour iron in a nitrate/phosphate ratio of 650/1, plants will grow. Of course not. They will simply die, because the precipitation of a huge proportion of iron into the sludge is inevitable in an aquarium. So, sludge with iron lingers in the soil, or in an external filter, and partially absorbs phosphates. Only if bound phosphates in the soil are available to plant roots, then they are not available in the filter. Then the filter is cleaned and, together with the sludge, the phosphate and iron leave the aquarium irrevocably.