It is important to control the amount of nitrate in aquarium water, since high levels can negatively affect the health of the fish. As a result, excess nitrates provoke the development of diseases that develop against the background of a weakened immune system. These include fin rot, white spot or fungal infection. But how can you check the nitrates in an aquarium and increase them if necessary? This will be discussed in more detail in this article.
Nitrates in the aquarium
Why does it arise?
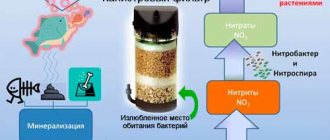
If we take into account all the nitrogen compounds that arise as a result of the vital activity of organisms, then nitrates are considered the safest of them. NO3 is formed due to the activity of some nitrifying bacteria that take part in the processing of ammonia (ammonia is released by aquarium inhabitants). Nitrates themselves do not threaten the lives of most aquarium inhabitants. On the contrary, they are directly involved in the growth of aquatic plants.
Nitrogen cycle in an aquarium
But the absence of a serious threat to fish does not mean that there is no need to monitor the level of the substance in the water. The fact is that increased nitrate content negatively affects some invertebrates and large fish . Therefore, the more fish there are in an aquarium, the more NO3 is released. Excessive feeding of fish can provoke an increase in the level of a chemical compound in the water. Because of this, organic matter accumulates at the bottom of the aquarium, which increases bacterial activity.
The more fish there are in an aquarium, the more NO3 is released
Note! Infrequent water changes and/or cleaning of the aquarium also contribute to increased nitrate levels.
It is important to clean your aquarium regularly
“Three pillars” of the nitrogen cycle. Part 3. Nitrate
Nitrates, produced from nitrites by hard-working bacteria, are traditionally considered safe for fish by aquarists. Indeed, if we remember the horrors that happen to a fish’s body under the influence of acutely toxic ammonia and nitrites, then nothing seems to happen at all from the presence of nitrates in water, even in much larger quantities. But is it? Alas, this is not the case, and underestimating the harmfulness of nitrates to the health of aquarium inhabitants is a very common belief.
So, let's take a closer look: by attaching just one oxygen atom to the nitrite molecule (that is, from a chemical point of view, by oxidizing nitrite), bacteria living in the soil and filter fillers save fish and other aquarium inhabitants from the danger of nitrite poisoning, making it The poison is another compound - nitrate. Aka NO3. The appearance of nitrates in the water of a freshly started aquarium is a good sign: this is a signal that the biofiltration system is working as it should, and all types of nitrifying bacteria have proliferated in minimally sufficient quantities (and different microscopic creatures are “responsible” for the different stages of nitrogen processing in water - for each of them tastes good about one substance). The discovery of the first, initially small, concentrations of nitrates is a significant reason for the aquarist to breathe easy. Soon, very soon, it will be possible to switch to the traditional scheme of caring for this “jar” and not monitor its well-being so closely: within a certain framework, it will now look after it itself.
Usually, a little nitrate is detected at first - about 5 or 10 milligrams per liter. Let us remember: for ammonia such concentrations would be completely prohibitive, but for the vast majority of fish and invertebrate species kept in aquariums these concentrations cannot even be called high. The rule works: the further along the nitrification chain our compound is located, the less toxic it is. Nitrates do not cause any pronounced symptoms of illness in fish, even if they are contained in water in “horse” concentrations. Should we conclude from this that they are not dangerous at all? No. The danger lies in the fact that these compounds affect the fish organism in a very specific way, and this effect is extended over time over months, and sometimes years, and goes unnoticed.
Now let's digress and imagine what happens to nitrates when they end up in aquarium water. The bacteria oxidized the nitrite molecule to form nitrate. And then what? So will he float in the water, or will he also turn into something? He has several paths. Let's look at them briefly to understand how you can influence nitrates in aquarium water if too much of them suddenly accumulates there.
The first way.
It is traditionally believed that in the nitrification chain this link is the last, and the process ends there. Indeed, this is true - but still this is only “half the truth”, since there is another process called denitrification. It is also carried out by special bacteria and consists in the decomposition of nitrates dissolved in water into two substances - oxygen and nitrogen. Each of them will no longer cause any harm to either fish or invertebrates. On the contrary, these gas molecules will either become involved in some new biochemical processes occurring in the aquarium, or simply evaporate from it. Both options can no longer be tracked in any way, and these processes, in general, do not have any significance for the aquarist - there is no point in trying to track something that cannot be tracked, even if there is no potential threat from it and cannot be.
So - do we have a happy ending? All that remains is to entrust the removal of nitrates to denitrifiers - and that’s it? Unfortunately, with these bacteria things are not so simple. Denitrification processes in an aquarium take place on a much more modest scale than is required for us to fully remove nitrates from water. On “fishing” forums, pros often have long conversations about establishing this process in home reservoirs, and the seeker will certainly get a lot of useful and interesting information from reading these posts. However, it is impossible to say that a simple and accessible way for every beginner has been found to reliably establish (and then control) the process of decomposition of nitrates into nitrogen and oxygen. Please note, this does not mean that it is in principle impossible to establish it! And yet, today we will not consider it - this is a topic for another, separate and much more complex material, richly flavored with Latin names and chemical formulas. For now, let’s just say that relying on denitrifying bacteria as “get rid of” nitrate formed in aquarium water is not an option. They will not help us fully cope with the problem.
The second way.
The idea that there are other “eaters” in the aquarium besides bacteria is not so unusual. The plants that we place in the underwater world behind glass are capable of absorbing nitrates with great appetite (and they also like the other nitrogen compounds that we talked about, even more), so in comparison with the nitrifying bacteria discussed above, ours green assistants are already much more effective. Therefore, the problem of water depletion in stable functioning herbal aquariums does not arise - there, on the contrary, sometimes it is necessary to add them specifically for sufficient nutrition of the plants. But this case is also beyond the scope of our conversation today - we will talk specifically about “overdose”.
It is known that different plant species “eat” nitrogen at different rates; The most “voracious” of them have also long been noticed (they are also usually the fastest growing and rapidly reproducing) - such as, for example, the ubiquitous hornwort or the well-known duckweed, which sometimes breeds in aquariums beyond all measure, forcing them to constantly clear the surface. Lower plants also like nitrates - just when there is an excess of them, algae (all these “strings”, “beards”, green dots on glass and stones) often begin to multiply in huge quantities. Let me note right away that this sign cannot be considered to clearly indicate an excess of nitrates: there may be other reasons for an algae outbreak in an aquarium. And it would be even more accurate to say that this reason is extremely rarely single, and if this happens, the situation will have to be corrected by some more thoughtful and large-scale actions than a simple mechanical decrease in the nitrate content. But in this case there is undoubtedly a reason to do a test for nitrates and check their concentration. Do it, and the result will explain something.
The third way.
This scenario can be called “reverse”. Nitrate appeared by attaching another, third oxygen atom to the nitrite molecule, so it can give it back. This happens in stagnant, anaerobic, i.e. areas of the aquarium deprived or almost deprived of oxygen, especially if there are already a lot of nitrates in the water (this also happens with small quantities - but the nitrite formed during the chemical reduction process is too small for it to harm anyone in the aquarium). You understand that we don’t need this scenario at all. Therefore, the issue of a good supply of oxygen to all areas of your aquarium is very important and is also on the agenda when we talk about combating increased concentrations of nitrogen compounds in water.
...This is approximately the situation with the possible utilization of nitrates in an aquarium. The thought immediately arises: well, plant a lot of plants - and you won’t know grief! They will absorb all the excess and you're done, beauty! But, of course, not everything in life is so simple and smooth. Some people don’t like a densely planted aquarium, but rather a “rocky” design. Some people simply don’t have a large number of plants and have nowhere to get them, or they bought fish that regard them as an appetizing “salad” for dinner, or... there can be many reasons, even to the point that the plants themselves are not always in this condition when they are able to actively feed. Be that as it may, there are still many situations where this simple and most natural solution is unacceptable.
What then?
There is one more way left - mechanical. We change the water in the aquarium, removing some of the nitrates along with it and thereby reducing their concentration. Water changes in an aquarium are needed for many reasons; we won’t touch on others here (this is a topic for a separate article) - but this alone is enough to do it regularly. The question of the frequency of these manipulations and the volume of replacements is so individual for each specific case that there is no point in giving any general “golden norm” either - it depends on too many factors. Let’s just say for now that most often in an “average” aquarium they replace somewhere around 20-25% of the volume per week, sometimes dividing this amount into two steps for species that are especially sensitive to changes in water parameters.
In an aquarium where biofiltration has already worked stably, nitrates from nitrites are constantly formed, since the vital activity of fish that release nitrogen into the water also does not have pauses (although it is activated in accordance with the feeding schedule). Ideally, some of the nitrates are eaten away by the plants, and some are removed by you yourself during replacement. Then, for a long time, the concentration of these compounds in the water remains at a stable level, moreover, low and quite acceptable for the vast majority of residents of indoor ponds.
But when this balance shifts towards the accumulation of nitrates in the water, then it turns out that “trouble crept up unnoticed.” During substitutions, you output less than necessary, and the excess accumulates with each week of such insufficient substitutions. The water in such aquariums is usually called “old”, and you can even recognize it visually - it has a characteristic, more or less pronounced yellowish tint. It arises due to a whole complex of substances dissolved in water, including humic acids, phosphates, and our friends - nitrates, and much more. All this occurs in the process of decomposition of fish feces, food crumbs not found by pets, plant debris, or the release of coloring substances from driftwood that is not well prepared for placement in the aquarium. Having noticed this yellowness, it is also useful to control the situation using an accurate and reliable tool - an aquarium test. He and only he will tell you exactly how many nitrates are contained in this water. Maybe the driftwood really colored it? Or maybe there is a reason to bother with more active substitutions for another, more serious reason.
The use of nitrate tests has its own specifics, but in general there is nothing so complicated that any beginner would not be able to do it. Therefore, there is no need to be afraid of the word “reagents” - after all, millions of our fellow hobbyists calmly use them! Five minutes - and you have a specific answer to the question of how many nitrates are contained in the water of your aquarium. You won't be able to answer it yourself.
So how much nitrate can there be in aquarium water? As we have already mentioned, this substance is not as toxic as ammonia, ammonium or nitrite. The threshold concentration, exceeding which in the vast majority of fish causes chronic poisoning, is 40 mg per liter. There are species that are more sensitive, this is especially pronounced in wild domestic or imported fish, which never encounter nitrates under natural conditions. For them, it is advisable to organize the most comfortable conditions with the minimum possible amount of nitrates, no more than 10-20 mg per liter, and monitor this indicator regularly (like the others, if necessary).
Let us emphasize once again: if the dose of 40 mg per liter is exceeded, fish experience CHRONIC nitrate poisoning. It is not acute, it occurs vaguely and asymptomatically, manifesting itself only in slower growth and a total decline in immunity, with prolonged exposure it is practically irreversible even when transferred to clean, nitrate-free water. In other words, there are no symptoms of nitrate poisoning in and of themselves. But you see that the fish develop too slowly (although this does not always happen - individual specimens of particularly resistant species, and even with generous feeding, and in such conditions manage to grow up healthy), and most importantly - they get sick with everything, everything imaginable and unimaginable diseases, the pathogens of which only appear within a kilometer radius. And at the same time, they are extremely difficult to treat, if they are treated at all, they relapse for no reason at all, they die from trivial diseases, they react extremely painfully to any medications, giving even the most harmless drugs the most acute toxicosis up to a state of shock. It seems that the fish’s body is on the verge: “touch it and it will fall apart.” This is how it really is: it’s only in our opinion all these months while the fish lived in saturated water (and in some such reservoirs, too cramped and/or filled to capacity with large fish, there may be 200 or 300 of them mg per liter!), nothing special happened to her... and all this time her body was waging a grueling battle with constant poisoning, which took all the strength, all the reserves it had. Is it any wonder after this that he cannot bear even the slightest load beyond that? That’s why the fish’s health goes haywire, when it seems like “everything has been fine for so many years,” for example, with the unfortunate goldfish, whiling away her life in a 30-liter gas chamber, a victim of the stereotype “a goldfish should live in a round jar.”
When the stocking standards of the aquarium are violated, when the fish are fed too often and abundantly, when water changes are too rare and small, when biofiltration works stably in the aquarium, the fish are not acutely poisoned by ammonia and nitrites, but all this leads to the picture we described. The fish either die from the most unexpected reasons, trivial diseases or the use of completely gentle medications, or it all ends with another “bad disease” - mycobacteriosis, or fish tuberculosis. This is an extremely serious disease, manifested by a huge number of different symptoms, sometimes specific even to related species, and practically impossible to fully cure. At least, we do not know of any reliable cases of fish being freed from tuberculosis. It is not always possible to slow down its development. It proceeds to match the nitrate poisoning that provokes it: for a long time, for months, it undermines the fish’s health, bringing it to a state where its organs simply fail. The sufferer has no way back. Not the slightest chance.
As you can see, nitrate is not as harmless as is commonly believed. I also hope that the above descriptions are enough to make a convincing statement: it is worth spending a little money and five minutes of time to control the nitrate content in your aquarium and protect your pets from big trouble.
But what if you’ve done all this and find that your fish are living in high nitrate concentrations? The main thing is not to rush to save them right away and not to replace all the water with clean water at once. Remember: their health is already weakened, and any stress can cause the most unpredictable consequences. Therefore, you should proceed as follows: for two weeks, replace 10-15% of the water in the aquarium every day (no more!), while simultaneously deciding whether the fish are planted too densely in this reservoir, whether they are overfed, whether add plants. After a two-week “rinsing” and taking measures to improve the situation (possibly planting plants, reviewing the diet, relocating fish), you should develop a replacement schedule in which nitrates will stop accumulating in the aquarium. By the way, don’t be alarmed if during this “rinsing” the test indicator turns out to be the same - you’ve been changing the water for a week now, but there are still the same amount of nitrates! How can this be? Don't be scared and continue. This is the peculiarity of this procedure; in the end it will definitely decrease.
After emergency measures have been taken, the fish should be provided with “sanatorium” conditions that best meet their species requirements, fed with a variety of foods, and given vitamins. According to our observations, the rehabilitation period can last about 5 months, and during this time (and even after it - also not uncommon) unexpected problems can arise with the health of the fish; they can often get sick, refuse food, and “mope” for no reason. Be patient: the fish’s body is looking for reserves, trying to recover. He needs to be helped, providing comfortable conditions day after day, so that he has as much strength as possible to do this. Remember that he will also regard even a transfer to a “sanatorium” as stress! That is why the principle here is so important: “It is easier to prevent a disease than to treat it.” Paradoxically, it is much easier to “pull out” and restore a fish to normal after severe ammonia poisoning than after prolonged nitrate poisoning. And since it’s quite simple to prevent the latter, it’s better to check the quality of the water once every couple of weeks for prevention, than to then deal with the consequences that we talked about and see illness and death of your pets. By saying “no” to high nitrates, you will do a lot for the health of your fish!
Tasha, Mistes. Photo by the authors Sudogda, Krasnodar, January 8, 2012
NO3 norm
The recommended concentration of nitrates in aquarium water is 20-30 mg/l, but there are more sensitive fish that can only feel comfortable at 15-20 mg/l. If there is a strong deviation from the norm, the health of aquatic inhabitants can be severely impaired.
The consequences of deviation from the norm can be the saddest.
The consequences of decreasing or increasing nitrate levels in water include:
- the appearance of spots on the body;
- change in fish color;
- decreased activity;
- slowing down the growth of the body;
- loss of appetite;
- weakening of the immune system.
In rare cases, death may occur. But this mainly happens to organisms that are particularly sensitive to nitrates.
The harm of lowering or raising nitrate levels
Consequences for fish
If no measures are taken to identify, control and reduce the level of nitrates, the inhabitants of the reservoir can be killed. In case of nitrate poisoning in fish, the following are observed:
- Damage to the gills and respiratory system as a whole;
- Oxidation of blood hemoglobin;
- Acute poisoning;
- Hypoxia (death occurs in the advanced stage).
Please note that with prolonged and regular exposure of aquatic inhabitants to high levels of toxic substances, their immune system is suppressed and a predisposition to chronic diseases is formed.
How to check your nitrate levels
There are special tests to check the level of nitrogen compounds in water . But if no problems should arise in performing this test, then special skill is required to determine the result. Therefore, it is important to study the instructions before using the test. There are strip tests, electronic devices and drop tests. Each type is described separately below.
Test strips
The easiest test to use. It is enough to put it in water and, after waiting a little, compare the test result with the norm. In addition, such tests are inexpensive and can be purchased individually. Despite the many advantages, the test strip has some disadvantages. The main one is the low accuracy of the results.
Test strips
Drip tests
This is a special liquid that changes color when in contact with water. After checking, you need to compare the resulting shade with the proposed scale. Depending on the concentration of nitrates, the color may change. Drip tests perfectly combine quality and price, which is why they are very popular.
Drip tests
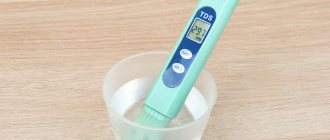
Electronic devices
Another type of test that can be used to determine the level of nitrates in water. The design of the device consists of a special probe and a digital panel. To check, you need to immerse the electronic test probe in water for a few seconds. The results will appear on the screen. The device quite accurately determines the amount of nitrogen compounds in water, and it is reusable. But due to the high cost, not everyone who regrets can afford this device.
Electronic test
Drip tests to check nitrate levels
Drip tests are called tests, for which you need to do the following manipulations:
- collect aquarium water into the provided test tube;
- add reagents to it in the form of solutions or powders according to the instructions for the specific drug;
- Shake the test tube, wait the required time and compare the color of the water with the scale.
The kits of such systems include all the necessary materials and tools - from test tubes and measuring spoons to the reagents themselves.
Drip tests are believed to have the highest accuracy, but this is not entirely true. Various factors may influence the results.
For example, if the nitrite content is more than 0.05 ppm, the nitrate test is guaranteed to be inaccurate. Also, a lot depends on the freshness of the reagents. Their effectiveness steadily and steadily decreases within 1 year from the date of production. But, despite these shortcomings, test systems of this type remain the most reliable.
Important! drop tests require strict storage and careful handling. Some reagents contain acetic acid, which can irritate the skin, respiratory tract and eyes. Others may contain flammable propylene glycol.
- Drip tests for NO3 can be purchased from various companies, VladOx, PRODAC, Sera, Tetra. Their cost is higher than that of test strips, but one package is usually enough for 60-100 tests.
How to reduce nitrate concentration
If a test of the aquarium water shows that the amount of nitrates exceeds the norm, then appropriate measures must be taken. It is worth noting that there are quite a lot of ways to reduce the amount of nitrogen-containing substances. The table below shows the most common and effective of them.
How to reduce nitrate concentration in an aquarium
Table. Ways to reduce nitrate in an aquarium.
| Way | Description |
| Aquarium charcoal | Coal does not remove nitrate, but only absorbs “dead organic matter,” which in turn provokes the appearance of nitrites. Later they are converted to NO3. The product is inexpensive but effective. |
| Partial water change | To reduce nitrate levels, you need to partially replace the aquarium water. About 25% once a week. It is also important to clean the aquarium and remove accumulated debris and organic particles. |
| Planting fast-growing algae | Special fast-growing algae . As they develop, they gradually absorb the chemical compound. You can buy algae at almost any pet store. |
| Nitrite blockers | Blockers are considered a temporary measure since they do not remove nitrogen substances from the aquarium, but only block them. But this is enough to take appropriate measures, for example, changing the water, improving filtration, etc. |
| Zeolites | The main function of zeolite is to suppress ammonia, which is eventually converted to nitrate. For greater effect on nitrate outbreaks, zeolites should be used in conjunction with other methods. |
Important! New water must be cleaned with a special filter before pouring into the aquarium. Alternatively, water can be passed through the resin.
Charcoal for aquarium
Why are NO3− compounds dangerous?
Substances have a third class of hazard, such as gasoline and manganese. The main risks are as follows:
Main table dispenser AquaPro 919H/RO (hot and cold water)
Main table dispenser AquaPro 929CH/RO (cooling/heating)
Floor dispenser AquaPro 311 (empty, without cooling)
- The connection has a cumulative effect, so the result can be extended over time.
- It is not noticeable to the ordinary eye. Without a chemical analysis, it will not be possible to know that the liquid is not suitable for drinking - it will not have sediment, a cloudy color or an unpleasant odor.
- It has a destructive effect on the body.
- Ordinary household filters do not help solve the problem; special cassettes will be needed.
How to Increase NO3 Levels
A low amount of nitrate is not the norm, so measures must be taken to increase it. Various methods can be used for this purpose. For example, adding chemical fertilizer. If fertilizers are added in small quantities to the water being replaced, this may affect the chemical composition of the water. Increasing the number of fish will also help increase the amount of NO3. When new inhabitants are added to the aquarium, organic residues will increase.
How to increase NO3 levels in an aquarium
Too high or low amounts of nitrates in aquarium water negatively affect the health of living inhabitants. Most often, organisms that are sensitive to the composition of water are affected. Therefore, it is important not only to feed the fish on time, but also to monitor the level of nitrate ions using the previously described tests.
Indications for cleaning
Unfortunately, it is not possible to determine the content of harmful substances “by eye”. The only direct purpose for filtration with 100% accurate results is chemical analysis readings. Therefore, it is very important to submit a sample to a laboratory. But if this is not possible, you can carry out a fairly effective method at home. There are special testers, as well as the use of the rivanol reaction.
Take 1 ml of drinking liquid, add 1 ml of saline solution and mix with 1 ml of rivanol solution. To do this, dissolve one tablet in 200 ml of 8% hydrochloric acid, heating it. If the resulting sample turns pink, then the concentration of harmful substances is too high for consumption.