People often turn to me for advice on how to make a beautiful, successful aquarium, but on the condition that “my water is not ideal.”
So, the first thing you need to start on the path to a healthy planted aquarium is to take care of the quality of the water in the aquarium. It is impossible to take water of any quality and make a masterpiece in it. Water must be created (prepared) and then used in the aquarium.
With the “use exactly what comes out of the tap” approach, achieving a successful aquarium is much more difficult. And often it is tap water that is the primary problem for the appearance of algae, fish disease, and death of shrimp. It's time to understand all the meanings. How to measure and control them. And in general, why is all this needed?
What do kN, gH, TS, TDS, TSS mean in water?
TS (total salts) = TDS+TSS is everything that is dissolved in water. Depending on the granulometric composition, we can distinguish:
TSS (volume of suspended particles)
The total content of particles whose size exceeds 2 microns. This could be fish waste, bacteria, rotted plant tissue. This is an instrumentally unmeasurable indicator that can be replaced by the “transparency\turbidity” parameter.
Such testing is not carried out in aquariums, except by eye, but for those who are curious, I will briefly tell you about testing water for the TSS indicator.
Water is poured through a special filter, after which the filter is washed with deionization water (from under the reverse osmosis filter). This is done so that the TDS readings do not affect the TSS readings. The increase in filter weight is then measured.
While working as an employee at a natural aquarium gallery, I noticed that many people commented on the extreme clarity of the water in the aquariums.
This can be attributed to the TSS parameter. This parameter is important when keeping small fish and shrimp. They have delicate gills that can become clogged with TSS particles, which can lead to their death.
TSS can be adjusted:
- mechanically using powerful filtration with external filters and internal filters with padding polyester filler.
- chemically with the help of special conditioners against turbid water, which deposit such large particles from the water column to the bottom. Then they need to be siphoned off from the bottom.
An abundance of suspended particles can be a result of overfeeding your fish, overcrowding of the aquarium (a lot of fish waste and, as a result, bacteria), death of plant leaves (which means something is wrong in the aquarium) or insufficient filtration in your aquarium.
From an aquascaping point of view, the abundance of suspended particles influences the appearance of algae on leaves, since these particles can settle on the leaves of plants and, therefore, act as a site (substrate) for algae growth.
The beloved moss of many may even die from the abundance of TSS on the leaves and stems.
Based on this, in professional aquascapes you can rarely see a large number of fish (especially large ones) and an abundance of moss at the same time.
The degree of filtration of an aquarium is assessed not only from the position of the volume of the filter media (media), but also from the power of the flow from the filter. Thus, the presence of a large number of suspended particles may also indicate insufficient flow in the aquarium. Don't forget to take care of this too. For example, wash the filter in a timely manner and change the fillers.
- In the case of a very overgrown aquarium, it may be a good solution to install a current pump. It has no filler, but only creates additional flow in the aquarium.
- Also, the more dissolved substances in the water, the lower the clarity of the water, which has a strong effect on the penetration of light into the water. And this is a direct relationship with the process of plant photosynthesis.
I think TSS has been sorted out.
TDS (volume of dissolved salts)
gH + kH + Nitrates + Nitrites + Chlorides + Chloramines + Sulfates + Phosphates + Ammonium + Carbonates + Bicarbonates + other compounds (fertilizers, water conditioners, fish medicines).
An instrumentally measured water parameter showing how many different chemical additives are dissolved in water.
For example, it can be used to understand how hard or soft the water is (but this is not the optimal parameter for such an analysis), how well the plants consume fertilizers added to the water.
Based on the amount of dissolved salts, the following categories are distinguished:
- Less than 1500 mg\l (ppm) - fresh water
- 1500-5000 mg\l (ppm) - brackish water
- More than 1500 mg\l (ppm) - sea water
A particularly high TDS value may be a sign of poor water quality.
- In the case of TSS, it is often enough for us to install a high-quality filter and there will be much less suspended particles in the water or there will be none at all.
- In the case of TDS, mechanical filtration does not work. Here, only the chemical component of aquarium filtration will help us.
pH value
The hydrogen index or pH of water indicates the amount of free hydrogen ions (H+), or more precisely the ratio of H+ and OH- (together they form the familiar formula H2O). Water itself practically does not dissociate into H+ and OH-, but since it contains a large number of dissolved substances (for example, salts of alkaline earth metals), some of them give a chemical reaction, shifting the pH value in one direction or another. If there are a lot of hydrogen ions in water, it is acidic, if there are few, it is alkaline.
Ionic composition of neutral pH value
The number of hydrogen ions (H+) is equal to the hydroxide ions (OH−)
Ionic composition of acidic pH value
The number of hydrogen ions (H+) is greater than hydroxide ions (OH−)
Ionic composition of alkaline pH value
There are fewer hydrogen ions (H+) than hydroxide ions (OH−).
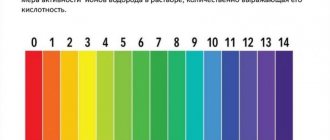
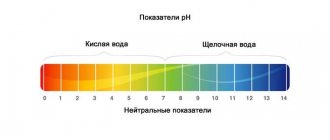
pH is measured on a scale from 0 (very acidic water) to 14 (very alkaline water). Unlike the general hardness of water, the designation of the pH value is the same in all countries, so there is no confusion.
Fish can live between 5 and 9 pH. The middle of the scale, number 7 (pH7), is considered a neutral value.
pH <5 - strongly acidic pH 5-6 - acidic pH 6-6.8 slightly acidic pH 6.8-7.2 - neutral pH 7.2-8.0 slightly alkaline pH 8.0-9.0 - alkaline pH >9 – highly alkaline
The pH in an aquarium is very unstable, especially in soft water. During the day, slight changes in one direction or another are possible. The daily fluctuations are most influenced by carbon dioxide produced by fish and plants (at night), and various organic wastes (excrement, uneaten food debris), which are actively involved in water oxidation.
As noted above, it serves as a buffer for pH, keeping it in a certain range of values. There is a direct relationship between them, which can be described in a table. The third indicator in the table includes carbon dioxide, since the degree of dissolution of CO2 also depends on the ratio of carbonate hardness and hydrogen index.
pH, KH and CO2 ratio table
The relationship between pH (pH) and carbonate hardness (KH) and its effect on carbon dioxide content
Why is pH important?
Along with temperature and GH, pH is of key importance when keeping aquarium fish, shrimp and growing aquatic plants.
Being from different biotopes, the inhabitants of the aquarium need a certain composition of water. For example, African cichlids from rift lakes have adapted to alkaline water, while fish from the Amazon, on the contrary, can only live at acidic pH values. Healthy plant growth requires nutrients that are most efficiently absorbed at low pH values (such as iron, manganese, boron, copper and zinc).
Why do you need to know TDS and where is it used?
The first and probably most important is the chemical purity of the source water. Almost all professional aquascapers use reverse osmosis filters to create their aquariums.
These are special filters that are installed in the water supply of your house or apartment, like ordinary household filters, with the only difference that you cannot drink such water and it is used for specialized purposes, one of which is an aquarium with plants.
Reverse osmosis filters purify tap water until it is almost distilled.
The degree of water purification is controlled precisely by the TDS parameter.
A reverse osmosis filter consists of prefilters: a mechanical filter and a carbon filter, as well as a membrane - the main element of the reverse osmosis filter.
Prefilters serve as membrane protection to increase its service life.
With a TDS from the water supply of ~300, the carbon filter cartridge is consumed within a week, the mechanical cleaning cartridge within two weeks, but the condition of the membrane can only be assessed by TDS.
The new filter purifies water from ~300 TDS to ~4. When the TDS value of water after the filter (usually the measurement is taken at the outlet of the membrane) begins to increase even taking into account new filters, it means that the time to replace the membrane with a new one is approaching.
Typically, filters with deionizing resin are installed after the membrane. It “finishes off” the residual TDS after the membrane from 3-4 to 0 ppm.
I recommend using exactly this water (0 ppm) in planted aquariums, because leaving TDS after osmosis at a value other than zero, we do not know what remains in this water, since we saw earlier that TDS consists from a large number of different components.
The condition of ion exchange resins is also monitored using TDS. Thus, when using reverse osmosis water, we control the TDS twice.
After the membrane filter and after ion exchange resins.
SpectraPure has recognized the need to measure TDS frequently and has built electronic TDS meters into its filters. Some filters from this company even have two of them.
What TDS is the best water to use in a planted aquarium?
This depends on the fertilizers you plan to use in your aquarium and the fish and plants you plan to use.
For example, fish from Lake Tanganyika live in nature in water with a TDS of ~400 ppm, fish from the Amazon River (for example, the well-known Neons - Paracheirodon axelrodi) with a TDS close to 0. So, TDS (here this can be equated to water hardness gH - what is it this will be below) is one of the determining factors of the future inhabitants of the aquarium.
Since reverse osmosis filters purify the water as much as possible, leaving only H20 molecules, which means there is nothing useful left in the water for plants. Thus, it is necessary to consider adding fertilizers with the widest range of macro- and microelements (spectrum, not concentration).
Here we can distinguish two large categories of fertilizers: those that are suitable for use in pure osmosis and those that are not.
Fertilizers from the following companies are exactly suitable for osmosis:
Tropica
ADA
Prodibio
The rest of the fertilizers are labeled “must try.”
I have never been shy about telling my clients “I don’t know, I need to try it,” because aquarium keeping is a hobby that does not have strict rules, all advice is just recommendations.
I recommend using fertilizers from other manufacturers with water TDS ~ 100 ppm.
How to prepare water with the required TDS?
- Restore water using gH+kH remineralizers
- Or mix osmotic water with tap water.
Many people use the second method because of its low cost: you can buy simpler fertilizers (which are not used in osmosis) and do not need to buy remineralizers.
I recommend the first method. It allows you to fully control the parameters of the source water.
What affects the norm?
The following factors are identified that influence the content of magnesium, calcium, and carbonates that change the composition:
Nature of water (tap, distilled, melt, spring, boiled).- Composition of tap water in a certain area.
- Types of inhabitants and products of their metabolism (fish, turtles, algae).
- The period during which the water is not changed and the aquarium is not cleaned.
- The presence or absence of water filtration.
- Decor (gravel, large stones, castles).
- The presence or absence of direct sunlight falling on vegetation.
Even if tap water in a certain area has excessive carbonate levels, they can be adjusted. This is important for aquariums where expensive fish species are bred.
What TDS does your aquarium need?
Make a list of the inhabitants of the aquarium:
- plants
- fish
- shrimps
- snails
See what TDS parameter is optimal for each of them (what it is in their nature - it may take a lot of time to find such information) and choose the average value. Some of the selected population may have to be abandoned.
If you plan to keep shrimp, take special care of the TDS value of the water you prepare. The minimum TDS value for keeping shrimp is ~100. Of course, there are cases of successfully keeping shrimp in water with a lower salt content in the aquarium, but this is the exception rather than the rule.
TDS is an instrumentally measured water parameter, therefore, using an electronic TDS meter, you can measure this parameter in your aquarium (and, for example, to measure this parameter in your water supply or after a household filter, water with high TDS is also harmful for humans) .
Ideally, at the beginning of the week and at the end (before changing the water), your aquarium will have the same value for this parameter. This will mean that the fertilizer is consumed in full and does not accumulate in the aquarium, which means that algae have only minimal chances to grow.
The body of fish contains water and they are in the water. Fish cells separate these two waters, but they are semi-permeable. Higher concentration (more dense) water will try to pass into lower concentration (less dense) water until they are equal.
If the fish could not somehow control this natural flow, they would either quickly dehydrate or explode. But fish are able to control this through osmoregulation, a complex series of chemical processes.
The kidneys primarily work to eliminate excess water, but another function is to store and reabsorb essential salts. Both processes work to maintain a specific balance of salt and water.
Thus, high osmotic pressure (caused by elevated TDS levels outside the fish's natural range) will overwhelm the fish with excess water and overload the kidneys, while low osmotic pressure (caused by TDS levels below the fish's natural range levels) will deprive the fish of water, causing dehydration.
TDS also affects the rate at which water penetrates fish through osmosis. “Clean” water moves through the fish cells very quickly, but water with some TDS will move more slowly. Fish use their kidneys to pump out this water.
The kidneys of fish that are found in hard water do not have to work very hard.
Softwater fish are built to live in water, which they quickly absorb to flush out toxins. A small tetra will urinate more than three times its weight every day.
But the higher the TDS, the harder it is for the fish to do this, so the toxins stay in their bodies longer.
Affect their physiology by causing stress and this will inevitably lead to a reduction in lifespan depending on the species and the difference between native range TDS and aquarium TDS.
Other compounds found in water are subject to control
3.1. Nitrates, nitrites and ammonia
These are compounds harmful to fish, the concentration of which must be strictly controlled. Particularly dangerous are nitrites and ammonia, which are destroyed in the filter by nitrifying bacteria to nitrates, which are used by plants during photosynthesis. These compounds arise from food debris, dead plant parts and are introduced into the aquarium along with fish excrement. Ammonium compounds cause an increase in pH. Excess ammonia can cause stunted plant growth.
Excess nitrogen compounds occur due to pruning, accumulation of excess silt, fish droppings and decomposition of plants and food debris. Lack of regular water changes in the aquarium and improper filtration also affect the concentration of nitrogen compounds in the water.
To prevent this, regularly replace a little water (every 2 weeks about 25% of the water), feed the fish in smaller portions (so that the food does not fall to the bottom and rot), clean the filters regularly (preferably with a water change - it is better to rinse the filter in removed water from aquarium, do not destroy the bacterial fauna that supports the filtration process). Never wash the filter in tap water) and do not overfill the aquarium (1 cm of fish = 1 liter of water). You can also introduce more plants that will use nitrates for photosynthesis, effectively reducing the amount of nitrate in the water.
3.2. chlorine
Chlorine is a compound used in the treatment of tap water (tap water). It is used to kill bacteria. It is also used to disinfect swimming pool water. As you know, excess chlorine in a swimming pool can burn human skin. The same thing happens to fish when we put them directly into an aquarium with water poured from the tap and not treated (not released). The amount of chlorine can be checked using testers available in stores. To remove it from the water, you can use commercially available products called antichlorites or let the water sit for a few to several hours.
3.3. phosphates
It is created in containers that are broken or when fertilizers containing phosphorus compounds are used incorrectly. This causes an increase in the amount of algae in the aquarium. The amounts of these compounds can be checked using ready-made test kits available at aquarium stores. They can be increased with potassium dihydrogen phosphate KH 2 PO 4 or by feeding the fish intensively with live and frozen foods. To reduce the amount, replace the water or use KNO 3 potassium nitrate.
3.4. iron
Both excess and deficiency are harmful. Excess is harmful to fish and some plants, and deficiency causes yellowing of plant leaves. To increase the iron levels in your water, you can use Chelat Fe. To reduce its amount, we will replace some of the water.
Relationship between TDS and gH (total permanent water hardness)
As we already know, TDS includes many different salts and by measuring only this indicator, we do not have enough information to understand what kind of water we have. Therefore, it is convenient to measure the indicators included in TDS, each separately (of course, if necessary).
gH is one of the main components of TDS - it is a combination of Calcium and Magnesium salts. They are usually represented by Sulfates and Chlorides. This is important when using osmosis water remineralizers. Pay attention that the salts are represented by sulfates.
When talking about whether water is hard or soft, it is the gH indicator that is meant. Therefore, the hardness of water containing only remineralizer salts (for example, freshly prepared water) can be measured using a TDS meter rather than a drop test.
The role of Calcium and Magnesium in a planted aquarium
Calcium is one of the most important elements for a planted aquarium, playing a very important role in the metabolism of aquatic plants. Calcium activates enzymes, is a structuring element for cell walls, affects the movement of water in cells and is an essential element for cell growth and division.
Calcium deficiency leads to slower growth of stems as well as roots.
Symptoms range from distorted, twisted young leaves to black spots on the leaves, and yellowing of the leaves at the edges is also possible.
Since calcium is not retransmitted to new leaves, symptoms of deficiency usually appear first on them, although not always. Pale spots or general leaf whitening (yellowing) may also be signs of calcium deficiency.
Calcium deficiency leads to disturbances in the functioning of roots and can cause iron poisoning.
- Magnesium is also a macronutrient in planted aquariums. Magnesium helps improve flowering; enhances green color; and may even help plants grow bushier. Its content is 0.2% of plant dry matter, and in some plants the concentration of magnesium in tissues is comparable to the concentration of phosphorus, the main nutrient.
- Magnesium is one of the components of chlorophyll. In addition, it activates the action of many enzymes. Some sources classify magnesium as a mobile element (from one part of the plant to another), so symptoms of magnesium deficiency are primarily observed in older leaves. However, the magnesium content itself is not as important as the Ca:Mg ratio.
According to reports from many aquarists, including Diana Wallstadt, the optimal Ca:Mg ratio is 4:1, but not less than Ca (20-30) - (5-8). Despite this, manufacturers in their fertilizers (not remineralizers) can use other Ca:Mg ratios, up to 1:1.
However, one aquarist from Argentina, having conducted many experiments with fertilizers and selecting optimal water parameters for aquariums with plants, claims that the ratio should not be 4:1, but, on the contrary, 1:4.
I have personally experimented with increasing the level of magnesium in water, adding enough to make the value 4 times greater than calcium, but did not see any drastic differences in the results. It is possible that rebuilding the aquarium to the new Ca:Mg ratio requires much more time than I gave it.
Interveinal (intercellular) chlorosis, sometimes in the form of dots, begins at the tips and edges, then spreads to the center without affecting the central vein of the leaf. Over time, symptoms of magnesium deficiency spread to young leaves.
Other sources suggest, on the contrary, that symptoms begin on young and middle leaves. Leaf areas affected by chlorosis may change color from yellow to brown (or even purple and red).
In cases of severe magnesium deficiency, necrosis and active leaf fall may occur.
Sometimes leaf fall occurs without preliminary symptoms.
There are sources that claim that young leaves can bend and their size can decrease.
Magnesium deficiency can be caused by insufficient supplementation or by high levels of calcium, potassium or sodium. Conversely, excess potassium or calcium blocks magnesium uptake, causing magnesium deficiency in plants.
From personal experience: for the last three years I have worked in the aquarium gallery @forms_of_life_moscow and there I used pure osmosis with TDS ~0 and ADA fertilizers, without additional remineralization of the source water. I did not observe the described problems with a lack of Calcium or Magnesium due to the fact that these fertilizers contain both Calcium and Magnesium. So, using the most complex fertilizers (ADA, Tropica, Prodibio), you can not add Calcium and Magnesium, they will most likely already be included in the composition.
Water in an aquarium with plants
Aquascape Promotion > Articles about aquascaping >
Water in an aquarium with plants
In the step-by-step instructions for creating an aquascape for beginners, you have already read about the water parameters in an aquarium with plants, which is the subject of an entire chapter of the instructions - Water Treatment . However, questions still arise regarding the selection of optimal water parameters in the aquarium and how to obtain them. This article will give not only pH and water hardness indicators for an aquarium with plants, but also describe methods for how to change these parameters if tap water has unsuitable water parameters for growing aquarium plants.
There are actually many options: tap water, spring water, rain water, boiled water, water after a reverse osmosis filter, distilled water, remineralized water. All this is water, but the essence is not in it, but in what this water contains. Water mainly contains minerals, the main ones being hardness salts (sulfates and carbonates of calcium and magnesium) and they are contained in different waters in different quantities. Sometimes there are too many of them, which interferes with many aquarium plants. And sometimes there are too few of them, which is also not welcomed by plants, because the minerals contained in the water work in exactly the same way as fertilizers for aquarium plants.
Most often, tap water is used for growing aquarium plants. It's convenient and economical. But we should not forget that tap water is often chlorinated and may be unsafe for fish in the aquarium. When changing water in an aquarium to eliminate chlorine, aquarists usually use special products that also bind heavy metals and contain vitamins to reduce fish stress, such as AQUAYER AntiToxin Vita. If tap water parameters are not suitable for growing aquarium plants, they can be adjusted. Methods for regulating water parameters will be described in detail in this article. Now let's figure out what the appropriate water parameters are.
Let's start with the main thing - the pH acidity of the water in the aquarium. The acidity indicator pH displays the proportion of acids and bases in water. Essentially, in an aquarium it all comes down to the ratio of one acid and one base. The base is carbonates, the amount of which illustrates the KH value (carbonate hardness). Acid is carbon dioxide - CO2, more precisely carbonic acid, which is partially formed when CO2 is dissolved in water. Carbonate hardness in an aquarium usually does not change. Therefore, the main factor influencing pH is the concentration of CO2. The higher the CO2 concentration, the lower the pH. But one more important point needs to be taken into account. Over time, other acids can accumulate in the aquarium as a result of natural biochemical processes (nitrification, etc.). Therefore, despite the concentration of CO2, the pH in the aquarium decreases over time.
What pH value should be maintained in an aquarium with plants? A pH value of 6-7 is suitable for most aquarium plants. However, it is not worthwhile to bring all types of aquarium plants under one general rule. Each plant has its own natural habitat and ideally in an aquarium you need to maintain the parameters to which the plant was originally adapted. Many plants prefer soft, slightly acidic water (pH less than 7), but there are species that are quite suitable for hard water with a slightly alkaline reaction (pH up to 8). For example, rotals and tonins thrive at a pH of 5.5 and below, but these conditions are detrimental to Hemianthus micranthemoides. Vallisneria, Elodea and many species of Echinodorus grow well at a pH of 7.5-8. But the pH range of 6-7 is a certain intersection point at which all these species grow acceptably.
The pH of the water in the aquarium affects many life processes of plants, in particular their consumption of nutrients. At the end of the article Diseases of aquarium plants, the dependence of the consumption of nutrients on the pH of the water is indicated. Taking this dependence into account, we can safely draw the following conclusion. Using the same composition of aquarium fertilizers at different water pH values will be perceived by plants as equivalent to using different compositions of aquarium fertilizers at the same water pH. In simple terms, if in order to maintain balance, the same fertilizer is used in the aquarium, then the pH reading for these purposes should be maintained at the same level. In this sense, using a pH controller to regulate the supply of CO2 has its undeniable advantages.
As mentioned in the previous chapter, KH - carbonate hardness (or alkalinity) affects the pH of the water in the aquarium. This means that KN is an important parameter for a planted aquarium.
Carbonate hardness of water is the amount of dissolved calcium and magnesium carbonates in water. However, there is water in which the value of carbonate hardness is determined by the content of sodium or potassium carbonate. In such cases, KH can exceed GH values, which confuses many aquarists. Therefore, it is more correct to call KN an indicator of alkalinity.
The optimal value of carbonate hardness for an aquarium with plants lies in the range KH 3-6. Over time, the water in the aquarium becomes acidified, so the KN in older aquariums may be higher than specified. But for the same reasons, it is better to avoid dropping the pH to values less than 3, since in the case of old aquariums the pH can drop below 6.
GH - the total hardness of water in an aquarium with plants is not as important a parameter as KH. But a low total hardness value can have a detrimental effect on aquarium plants. Why? General hardness is characterized by the content of calcium and magnesium salts.
Calcium and magnesium are needed by plants for growth and are macronutrients, like fertilizers. It follows that you should only worry about low overall rigidity.
Some types of aquarium plants begin to show signs of calcium deficiency already at GH 3. Therefore, it is better to maintain the total hardness value starting from 4 degrees, and to be sure, 6-8 degrees.
The most common way to soften water, namely to reduce both total and carbonate hardness, is to use water after a reverse osmosis filter. Pure reverse osmosis water cannot be used in an aquarium because it has GH-0 and KH-0. But if tap water is hard, then water for an aquarium with plants can be prepared by mixing tap water and osmosis according to the principle described in the Instructions in the chapter on water hardness in an aquarium. This method is suitable for large aquariums.
In the case of a small aquarium, or nano aquarium, not everyone is ready to purchase a reverse osmosis filter, which is larger in size and more expensive than the aquarium itself.
An alternative is the special AQUAYER pH/KH minus product, which reduces carbonate hardness and pH without changing GH.
If the choice is to use water filtered by reverse osmosis, then you need to know some nuances. As already mentioned, such water has zero parameters of total and carbonate hardness, which is unacceptable for aquarium plants. This water must be restored to the required hardness parameters. There are two ways to restore stiffness.
The first, mentioned above, is mixing this water with hard tap water. The preparation method is described in the Instructions in the chapter on water hardness in the aquarium.
The second method is remineralization with calcium and magnesium salts. There are many options for mixtures for remineralizing water after a reverse osmosis filter. Some mixtures contain calcium chloride simply because it is an easily soluble salt and easy to use. However, as the overall GH hardness increases, these salts also significantly increase the chloride concentration. Chloride in high concentrations inhibits the growth of plants in the aquarium and this effect is most noticeable on fastidious plants. In other mixtures, only sulfates are used and, although they do not contain chlorides, at the same time they do not restore carbonate hardness (CH), only total (GH). If carbonate hardness is not restored, under carbon dioxide (CO2) conditions, the pH of the water may drop below 6. Some plants may dissolve at a pH below 6. Also, with zero carbonate hardness, the pH may fluctuate widely, which can cause fish kills. The mixture of minerals Remineral GH/KH+ allows you to restore not only general hardness, but also carbonate hardness in an optimal ratio. In fact, its formula consists of the same calcium and magnesium salts that are found in natural water. At the same time, Remineral GH/KH+ also does not contain chlorides.
It is not always a task to reduce the water hardness in an aquarium for good growth of aquarium plants. In some regions, tap water, on the contrary, has very low hardness. In such cases, it is necessary to increase the total and/or carbonate hardness.
A simple but poorly controlled way to increase rigidity is to use marble chips. It consists of calcium and magnesium carbonates, therefore it increases both total and carbonate hardness. For a small aquarium, a small handful in an inconspicuous corner of the aquarium may be enough to raise the hardness by a few degrees. The uncontrollability of this method lies in the fact that the rigidity can rise to higher parameters.
A more controlled way, but also more complex, is as follows. To increase overall hardness, a mixture of calcium and magnesium sulfate can be used. For example, the following combination increases the total hardness (GH) by 3 degrees in 70 liters of water: 1) 4.8 g calcium sulfate CaSO4*2H2O. Approximately 5ml. You can measure it with a syringe. This salt dissolves slowly in water. 2) 2.8 g of magnesium sulfate MgSO4*7H2O. Approximately 3ml. You can also use a syringe. Baking soda can be used to increase carbonate hardness. The following amount of soda increases carbonate hardness (KH) by 3 degrees in 50 liters of water: 4.67 baking soda NaHCO3. Approximately 5ml. You can measure it with a syringe. If finding these components is difficult, then you can increase the total and carbonate hardness with the help of Remineral.
You may have questions after reading this article. You can always ask them on the forum in the section Water for an aquarium with plants.
Carbonate hardness dKH
And now a few words about the most difficult parameter of water - temporary hardness (carbonate hardness).
Here I will not write much about this parameter, since an in-depth description of it is a whole separate article.
dKh is the total content of carbonate and bicarbonate ions in water. And hardness dKh can only be called if it is calcium carbonate or magnesium carbonate, i.e. when carbonates are added, calcium or magnesium is added, released into the water when they react with an acidic environment.
It is correct to call this parameter “alkalinity”. This parameter is also called a buffer. Oddly enough, this is not related to the pH of the water, which indicates whether the environment in the aquarium is alkaline or acidic.
Alkalinity is the ability of water to maintain a stable pH (avoid pH fluctuations - this is why dKh is such an important parameter). This is important to consider when intensively supplying carbon dioxide.
Active saturation with carbon dioxide can lead to a sharp drop in pH level (shift to an acidic environment); to prevent this from happening, it is necessary to add a carbonate buffer dKh to the osmotic water. The higher dKh, the more stable the pH, but also the more carbon dioxide CO2 must be supplied in order to shift its value by the same amount (which is why dKh should not be high).
I wrote above that water can be remineralized using gH and kH salts, or only gH. How do you know which remineralizer to use?
If you use aquasoil soil (for example ADA Amazonia), it already contains a buffer, but not alkaline in the form of carbonates, but acidic in the form of humic acids, due to which the pH level will always decrease and remain at a value of ~6.7 (optimal level pH in the aquarium is 6.6-6.8) in an aquarium with such soil.
Thus, in aquariums with Soil type soil there is no need to additionally supply a buffer, and when using complex fertilizers with calcium and magnesium in the composition, there is also no need to add gH.
It has been noted that in natural waters the level of dKh usually does not exceed the level of dGh, but gH can be 1.5-2-3 times higher than dKh.
How to vary the hardness
Downward
Water can be passed through an osmotic type filter.
You can add peat to the filter or to the container for settling water.
You can buy distilled water in the store and pour it into the aquarium.
Instead of distilled water, it is permissible to use melt water from the refrigerator or natural precipitation. The main thing is that the liquid does not contain impurities and is transparent.
Water can be boiled for 60 minutes in an enamel bowl and allowed to stand for 24 hours.
Fast-growing vegetation (hornwort, vallisneria, nayas, elodea) softens the water in a natural way.
Increasingly
To achieve an increase of 4°dKH, 1 tsp should be dissolved in 50 liters of water. baking soda.
In order for both KH and GH to increase by 4°, calcium carbonate in a volume of 2 tsp is dissolved in 50 liters of water.
If you place shells from the sea in the aquarium, the hardness indicator will gradually increase naturally.
The hardness of the water inside the aquarium can be measured at home without drugs or expensive equipment. This method is called titration with a soap solution.
If 1 liter of water contains 10 mg of calcium oxide, this indicator can be neutralized with 100 mg of soap.
Laundry soap 60-72% should be crumbled.
You need to pour distillate into the glass (that is, melt water from the refrigerator, store-bought distilled water, or natural precipitation).
Soap crumbs are added to the liquid in exact proportions.
Pour 0.5 liters of water taken from the aquarium into another container and add a soap solution in doses of 100 mg, then shake.
Blue-colored flakes and bubbles should form on the surface of the liquid, which will disappear immediately. The soap solution should be poured into the container in doses until all the magnesium and calcium oxide are completely bound. The soap bubbles on the surface of the liquid should become stable and begin to shimmer like a rainbow.
The number of portions of soap solution that needed to be poured into the container to achieve the result should be multiplied by 2. The result of the multiplication will be an indicator of water hardness. If you act carefully, the experimental error will fluctuate in the range of +-1° hD.
The reliability of the result decreases at hardness above 12° hD. In this case, the experiment should be duplicated by combining 1 part of water from the aquarium with 1 part of distillate, and the calculation result should be multiplied by 2.