11/13/2019Aquarium0
Live foods are widely used among aquarists. They contribute to the beginning of the reproductive process of fish, help to show the brightness of the color and serve as an excellent source of nutrition for the fry.
The rotifer (Rotatoria) is one of the smallest species of freshwater worms. The nutritional value, as well as the positive effect on the growth and development of juveniles, has popularized this species.
- 1 Description and natural habitat
- 2 Mr. Tail recommends: variety of species
- 3 Life cycle features
- 4 Feeding and rearing
- 5 Reproduction and development, artificial breeding in an aquarium
Description and natural habitat
The bilateral multicellular animal Rotifer is distinguished by the presence of a ciliated formation at the anterior end of the body. With its help, motor processes and nutrition are carried out.
The habitat is not regionally limited. Depending on the species, there are 1,500 of them, they live all over the world, inhabiting fresh and sea waters, and thrive in moist soil. 600 varieties of the worm have been discovered in Russia. The variety of forms includes freely moving, parasitic and symbiotic types.
The rotifer looks similar to a seahorse, only its tail is much shorter and is called a leg. In most individuals, the body is oblong and is divided into three parts: the head; torso with internal organs; leg or hindquarters. There are several spherical types. The animal does not have respiratory organs; the entire surface of the body takes part in this process.
The largest reach 2 mm, the smallest - 40 microns. You can see an animal of this size under a microscope. This is interesting, due to the size of the worm, the structure of the tissue changes; the cells do not have clear boundaries and are interconnected by cytoplasmic bridges. An animal has a constant number of cells throughout its life. When unfavorable conditions arise, the rotifer falls into a peculiar state of conservation by dehydrating the body.
The worm is inactive compared to ciliates and is much smaller in size than crustacean larvae. Reproduction occurs quickly, and parents of different sexes take part in the process. The rotifer has an unusual life cycle, divided into sexual reproduction and unisexual reproduction.
Rotifers mainly feed on tiny algae; they are also known to be predators.
Taxonomy and naming[edit]
See also: List of double-sided animal orders
The Reverend John Harris first described rotifers (specifically the bdelloid rotifers) in 1696 as “an animal like a large grub, which could be compressed into a spherical figure, and then stretched out again; the end of its tail appeared with pincers similar to those of an earwig." [2] In 1702, Anthony van Leeuwenhoek gave a detailed description of Rotifer vulgaris,
and then described
Melicerta ringens
and other species.
[7] He was also the first to publish observations of the resurgence of certain species after desiccation. Other forms were described by other observers, but it was only after the publication of Christian Gottfried Ehrenberg's Die Infusionsthierchen ALS vollkommene Organismen
in 1838 that rotifers were recognized as multicellular animals. [7]
About 2,200 species of rotifers have been described. Their taxonomy is currently constantly changing. One treatment places them in the phylum Rotifera with three classes: Seisonidea, Bdelloidea and Monogononta. [8] The largest group is Monogononta, with about 1,500 species, followed by Bdelloidea, with about 350 species. There are only two known genera with three species of Seisonidea. [9]
Acanthocephalans, previously considered a separate phylum, have been shown to be modified rotifers. The exact relationships with other members of the phylum have not yet been decided. [10] It is possible that acanthocephalans are closer to Bdelloidea and Monogononta than to Seisonidea; the corresponding names and relationships are shown in the cladogram below.
| Monogononta | |
Rotifers are strictly speaking limited to Bdelloidea and Monogononta. Rotifers, acanthocephalans and Seisonida form a clade called Syndermata. [eleven]
Etymology[edit]
The word rotifer
comes from the Neo-Latin word meaning "wheel-bearer", [12] due to the crown around the mouth, which in coordinated sequential motion resembles a wheel (although the organ does not actually rotate).
Mr. Tail recommends: variety of species
Aquarium enthusiasts use rotifers as food for fish and fry, so they mainly breed small species, 0.5 mm in size.
Let's look at the features:
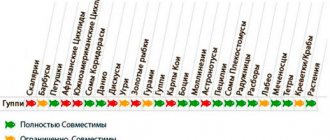
- Asplanchna priodonta - grows up to 1.5 mm, belongs to large varieties, has an incomplete spherical body shape. Reaching its maximum size, it turns into a predator, eating its own kind and ciliates. The worm is constantly in motion, moving through the water column with its mouth opening forward and without rotating. There is no color, the body is almost transparent. The nutritional properties of the species are weak; they are used as an intermediate product, transferring the fry to adult types of food. Combine with more useful ones.
- Brachionus calyciflorus - sometimes grows up to 0.25 mm in length. A sexually mature female is not so large, the body size is 0.6 mm. The short life cycle (21 days) forces the animal to start the reproduction process every 12 hours. This species feeds exclusively on plants. The value of the culture is high; it is widely used for feeding young aquarium fish.
- Brachionus plicatilis - grows up to 0.27 mm. It tolerates fluctuations in the chemical composition of the liquid well and survives at a maximum salt level of 90%. Eats exclusively phytoplankton and bacteria. The lifespan of males and females is different, females up to 14 days, males - 3 days. For reproduction, warm water is needed +17…+36 °C. The eggs settle to the bottom, where they hatch after a while. It is optimal for feeding fish, reproduces quickly and provides the maximum amount of nutrients. Aquarists treat this species with special respect.
- Brachionus rubens is a symbiont that lives in fresh water, measuring up to 0.29 mm in size. Lives and feeds by moving on daphnia, but cannot swim. The cycle from birth to death is 17 days, every 12 hours the female lays eggs that ripen at a temperature of + 22 ° C during the day. Herbivorous species.
- Philodina acuticornis - grows up to 0.5 mm, inhabits the silt of the bottom of artificial and natural reservoirs. Philodines move slowly by crawling or swimming, thus obtaining food. The animal has a cone shape, wide at the head and tapering towards the base of the legs. The diet consists of bacteria and algae. The long-lived worm reproduces for almost a month at a temperature of +24...+27 °C. The female lays 50 eggs.
Feeding fry with rotifers
Rotifers are an excellent food for raising fry. The “children” of many fish species are evolutionarily adapted to feed on these representatives of lower animals. Rotifers, which are a type of lower worms - the main component of zooplankton, are distributed everywhere, which means that the fry will find food in any body of water.
In nature, rotifers constitute a significant mass of zooplankton. Studies of freshwater lakes and small tributaries of rivers by Kazan Federal University researchers O.Yu. Derevenskaya and E.N. Unkovskaya showed that, in percentage terms, zooplankton consists of:
— Rotifers from 47% to 64%;
— Cladocera crustaceans – from 34% to 44%;
— Copepods from 19% to 24%.
Freshwater rotifers are present in the aquarium, usually gathering in places where their usual food is concentrated - bacteria, small ciliates, that is, where the decaying remains of parts of aquatic plants, excrement, and bottom silt are chipped off.
Representatives of the rotifer type are perfectly adapted to changes in the external environment. In laboratory studies, their eggs remained viable after many years of drying, cooling to -270°C for four hours, heating at +100°C for five minutes, and the living organisms themselves perfectly withstand drying, falling into a state of suspended animation. Thus, during the rainy seasons, when water temporarily fills various gaps in which spawning occurs, by the time the larvae emerge and transform into fry, nature prepares a generous table for the new generation in the form of rotifers.
Nature also made sure that fry with different sized mouth openings could use rotifers. The sizes of rotifers range from 0.03 millimeters to 2 millimeters; the sizes of the juveniles of these worms are also varied, depending on the stage of growth from the moment they emerge from the egg and reach adulthood.
Rotifers live in both fresh and salt water bodies. Not only species living in sea waters are known, but also intermediate species living in waters with a high content of NaCl and mineral salts.
Rotifers are an excellent starter food not only because they contain a fairly high amount of protein - 30%. This type of food is a source of natural low-molecular peptides, as well as free amino acids, which makes rotifers an easily digestible food for aquarium fish larvae in the early stages of development.
The advantages of rotifers as food for fry include their labile, that is, rapidly changing chemical composition. The process takes only a couple of hours and depends on the type of food available to the rotifers. This feature makes it possible to formulate exactly the food that meets the needs of the fry of a particular fish species.
Studies by Gatesoupe, 1991, showed that treating rotifers with an antibiotic immediately before feeding increased larval survival. Treatment with antibacterial substances inhibits the growth of pathogenic bacterial species from the Vibrionaceae family, which are fatal to most fry. Generally speaking, there is still a lot of uncertainty on this issue. The only fact that has been accurately established is that most of the bacteria that cause fish diseases are safe for rotifers and the latter can serve as their carriers along the food chain. At least, fish breeders on an industrial scale do not yet practice methods of enriching rotifers with antibiotics before feeding.
The modern approach to rotifer nutrition includes strategies aimed at enriching:
— essential fatty acids for fry;
- proteins;
- vitamins.
In this case, two strategies were formed:
- Japanese, in which enrichment is carried out by feeding rotifers with specially developed chlorella-based pastes;
- European, which is based on feeding with special industrially produced mixtures
Japanese is more preferable if the task is to enrich protein, European - if it is necessary to enrich with essential fatty acids. For example, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which under natural conditions intensively accumulate in fish immediately after hatching and ensure the normal formation of a neural network in the larval brain.
The following industrially produced mixtures for breeding rotifers have proven to be very productive:
— Culture Selcow® (CS);
— Protein Selcow ® (PS);
— DHA Culture Selcow ® (DHA-CS).
These feeds fully provide rotifers with food enriched with Ώ-3 polyunsaturated fatty acids, DHA, and EPA for feeding the fry.
Since rotifers cultured with yeast are produced with virtually no vitamin C, to overcome this deficiency, it is necessary to introduce chlorella or derivatives of this water-soluble vitamin into the feed, for example, ascorbyl palmitate, which the rotifer enzyme system converts into full-fledged vitamin C, which then enters the body of the fry. The presence of ascorbyl palmitate in just 5% concentration in the emulsion, in which the rotifers are kept for 12 hours before desalination to enrich with ascorbic acid, leads to an increase in the vitamin C content in rotifers from 150 mcg per gram of dry weight for those cultured with baker's yeast and 1000 mcg for those cultured with chlorella to 2300 mcg per gram of dry weight of Brachionus, treated by aging in an emulsion with ascorbyl palmitate. In this case, the vitamin level is maintained for 24 hours after removal of the emulsion.
It is important to note that rotifers are distinguished by their peculiar attachment to their habitat, that is, to water. If live rotifers were purchased as a starting culture, then for subsequent cultivation the composition of the water must coincide with the original one. If eggs were purchased, subsequently, after receiving live individuals, the composition of the water should not be changed.
Photo 2
Cultivation of rotifers
To cultivate rotifers, vessels with a capacity of three to one hundred liters are used.
It won’t be difficult to get a starter culture. Since rotifers lay eggs on molds, online stores have plenty of offers for the sale of such products. If for some reason you cannot purchase the required rotifers there, you can contact a fish farm, since rotifers are widely used for feeding table fish fry, or an organization that monitors the cleanliness of surrounding water bodies - rotifers are an excellent test for water purity. Rotifers are also bred in the laboratory in educational institutions where they study biology for practical training.
Subject to optimal cultivation conditions, it is possible to achieve a population density of rotifers of up to 300 specimens per milliliter, and even higher when using modern techniques.
Optimal cultivation conditions will include:
- The nature of the lighting, it should be lateral and not very intense, lasting eight hours a day;
- Temperature conditions - rotifers live in a fairly wide temperature range, from 15°C to 35°C, but the optimal temperature for them is 26°C - 28°C;
- Rotifers can withstand an increase in the acidity of the environment to 9.5, but the optimal pH for cultivation is from 7.1 to 7.6
Photo 3 Brachionus plicatilis
Cultivation of the saltwater rotifer
For zoologists, this saltwater rotifer is a typical representative of the taxonomic type of these animals. It feeds on phytoplankton, but if such food is not available, yeast, for example, is quite suitable as food for it.
Saltwater rotifers used in aquarium farming are a universal food in the sense that they can be fed to both fry of freshwater species of aquarium fish and marine species.
Br. Plicatilis is a saltwater rotifer, the breeding of which is practiced not only by aquarists, but also in large fish breeding enterprises engaged in the cultivation of not only aquarium, but also table fish species, such as cyprinids.
In laboratories and fish farms, salinity is maintained at 25%. This is achieved by adding a tablespoon of NaCl (it is better to take regular salt, not iodized) per 1 liter of water or by adding 83 ml of a supersaturated solution (300%) to the same amount of water.
A higher yield of rotifers can be achieved by regularly replacing the water with rotifers (1/3 of the volume) with the same water (83 milliliters of saturated salt solution per 1 liter of scenedesmus culture). The addition of algae accelerates the rate of development and reproduction of rotifers and the laying of eggs.
When cultivating rotifers, the choice of scenedesmus and chlorella as a food source is determined not only by the nutritional qualities, but also by the size of these microalgae. The oral apparatus of rotifers is designed in such a way that only particles not exceeding 15 micrometers are available for nutrition. The size of chlorella is 4 micrometers, the size of the cells that baker's yeast has is 6 micrometers.
Baker's yeast is used for yeast feeding. If alcoholic yeast is used, then it is pre-frozen in the freezer of the refrigerator, first thinly sliced and kept until it acquires a dark brown color. If you use fresh alcoholic yeast, the rotifers will die.
A high number of rotifers is achieved at a feeding rate of 40 milligrams per 2 liters of dry biomass.
For yeast, this ranges from 100 milligrams per liter to 1 gram per liter, depending on the intensity of culture development.
Many fish farmers determine the sufficiency of food availability by the transparency of the culture medium. This is not done “by eye”, subjectively, but using a Secchi disk. You can make it yourself. It is enough to cut out the bottom of a white plastic cup, divide the surface into four sectors and paint the two opposite ones black, and then attach it to a rod with centimeter marks along the length. As in limnology, transparency is measured in centimeters.
Photo 4
When cultivating, they try to maintain transparency at a level of 20 to a maximum of 25 centimeters.
If you want to get rotifer eggs, proceed as follows. Stop adding feed and reduce the temperature to 14°C. If intensive egg formation is not observed, the temperature is reduced by another 2 degrees. The lighting is removed. After a noticeable decrease in the number of rotifers, the culture water is drained, leaving a sediment formed at the bottom of the cultivator vessel, it is filtered using a paper filter, then dried in the dark in air and stored together with the filter.
Subsequently, it is easy to breed new rotifers from the eggs obtained in this way. In order for the process to be as successful as possible, the eggs must be frozen for a month or two before incubation at t = - 5°C -10°C. You can do without this stage, but the number of emerging rotifers will be slightly smaller.
In fish farms, for incubation, Brachionus plicatilis eggs in an amount of 1 gram per liter of 25% salt water are placed in a cultivator and a weak air blow is created. After a day or two, the rotifers begin to hatch from the eggs. You can visually monitor the normal development of eggs and the yield of future food. Empty shells will float to the surface, and developing eggs will sink.
The rotifers are fed immediately, introducing microalgae and yeast even before incubation begins.
In order to select sexually mature rotifers, gas No. 64 is used; if total selection is carried out, gas No. 76 is used.
To prevent the rotifer from immediately dying in the fresh water of the aquarium with the fry, it is first desalinated, reducing the salt concentration to 3%. To do this, gradually, drop by drop, 800 milliliters of aquarium water are added to the cultivator throughout the day. Most aquarists use medical droppers for this.
Photo 5
How to feed fry with saltwater rotifers
Selection of rotifers and transferring them to fry has its own subtleties. After desalination, the rotifers are caught with a net from the mill gas and filtered without removing them from the salt water. They do it this way. I place the vessel for selecting rotifers at a level lower than the cultivator and place a net or sieve in it so that ¾ of its vertical size is immersed in water, then select the rotifers from the places where they accumulate in the cultivator using a siphon and direct the stream of water so that it passed through the gas net.
Photo 6 Brachionus plicatilis ready for feeding to fry
Then the net, without allowing the water to drain, is transferred to a container with fresh water, rinsed to remove the remaining saline solution, and again, without allowing the water to drain, transferred to a container with larvae. If you allow the water to completely drain, the rotifer will clump and quickly die, bringing instead a benefit to an increase in nitrite levels.
When served to the fry for lunch using this method, the desalinated rotifer does not settle to the bottom in fresh water and lives from three hours to a day.
A properly installed sprayer in a nursery aquarium will promote uniform distribution of rotifers throughout the volume.
When fed with a desalinated rotifer, it does not settle to the bottom in fresh water and lives from 2 to 3 hours to a day. The sprayer in the container of the larvae helps to distribute the food evenly throughout the volume.
They give desalinated rotifers as the fry eat them, that is, four to eight times a day.
Simultaneously with the rotifer, the ciliate slipper is introduced to the larvae, which on the one hand is a constant feeding when the introduced rotifers settle to the bottom, and a new portion has not yet been introduced. On the other hand, ciliates perfectly purify water from bacteria.
As the fry grow, in the intervals between the introduction of rotifers, they begin feeding the fry with vinegar nematodes.
Photo 7
Freshwater rotifers
Cultivating freshwater rotifers is more difficult than saltwater rotifers, since ciliates, other protozoa and microorganisms that compete with rotifers for food and oxygen are often brought into the cultivator with dust, etc.
On the other hand, it is preferable to breed freshwater rotifers than saltwater rotifers, since freshwater rotifers do not die in an aquarium with fry, which ensures uniform and constant nutrition for the fry. At the same time, the aquarium is cleansed of bacteria that the rotifers eat.
Several types of rotifers are cultivated to feed the fry. Since among the variety of rotifer species in fresh water bodies, representatives of the family Brachionidae are present, and in artificial cultivation, preference is given to representatives of this family.
Photo 8
Brachionus rubens and Br. caluciflorus
These rotifers, living in fresh water and used to feed fry, are similar in appearance to saltwater rotifers, but their sizes are somewhat smaller, so they are an excellent food for neons and characins, for lalius and gouramis.
Unlike the saltwater rotifer Brachionus plicatilis, these freshwater rotifers have extensions on the front and back of their bodies. There is a certain connection between the length of the outgrowths and the amount of food available in the culture. If there is insufficient food for rotifers, the outgrowths lengthen and vice versa. This is because such outgrowths slow down the movement of the animal, which makes it easier to filter out absorbed particles.
Photo 9 Br. caluciflorus in case of nutritional deficiency
An interesting behavioral feature of Brachionus rubens in natural conditions. When there is a lack of food, this freshwater rotifer attaches its foot to the shell of the daphnia and, traveling with it, filters out food particles without expending extra energy.
Photo 10 The arrow indicates Brachionus rubens attached to daphnia.
It is also important for aquarists that the larvae of these rotifers are comparable in size to ciliates and have a higher nutritional value. Their size is from 0.1 to 0.3 millimeters.
There are descriptions of growing Br on forums. caliciflorus together with algae in complete darkness three t = 33°C - 37°C. Such conditions provoke parthenogenetic development of the rotifer, that is, a higher food mass. The method involves growing for 4 days, after which the culture is recharged. The disadvantages of the method include the fact that at such a high temperature and in the dark, not only the rotifers, but also the algae themselves breathe intensively. If you miss the moment due to a lack of O₂, the excessively proliferated rotifers will die. If you install a blower, then under conditions of intense water movement, the rotifers attach to the walls of the cultivator, shrink and stop feeding.
Some aquarists successfully solve this problem by cultivating rotifers in the dark together with Scenedesmus in low, very wide flat cuvettes. To select rotifers, they are attracted by illuminating one of the corners of the cuvette with a flashlight. If you plan to feed freshwater rotifers with yeast, this method is not used, since in such vessels it is almost impossible to maintain the yeast in suspension.
It should be taken into account that freshwater rotifers of these species feed not so much on algae as on bacteria, which rapidly multiply on the surface of dead algae cells. Thus, good results can be obtained by keeping the phytoplankton in the dark for several days (before sedimentation begins), and then, after shaking it well, feed it to the rotifers.
Good outbreaks of the number of rotifers (such cases are described in the literature for Br. rubens) are achieved by keeping them in semi-darkness with feeding a mixture that consists of sludge based on dead algae from the phytoblock, a culture of living scendesmus, as well as bacteria obtained from the decomposition of a boiled egg , carrots, meat broth, milk. Temperature - 28°C.
Twilight ensures a constant supply of O₂ into the cultivator water as a result of photosynthesis, as well as the adsorption of bacteria on the surface of living algae cells.
Strong light when cultivated on algae is dangerous for rotifers, which die from the oxygen intensively released in their intestines by the absorbed algae before digestion.
The next method of continuous cultivation is the use of anaerobic bacteria to feed rotifers.
The reproductive process of rotifers requires constant monitoring to prevent the transition to sexual reproduction, which will result in resting eggs and the death of the rotifers themselves. This can happen when oxygen levels are low, or with overfeeding or underfeeding.
Temperature range for ripening Br. rubens and Br. caluciflorus is quite wide - from 22°C to 32°C. At these incubation temperatures they ripen within 24 hours.
Lifespan of Br. rubens and Br. caluciflorus from 4 to 17 days.
The females of these rotifers lay three to twelve eggs every twelve hours. In companies engaged in the industrial breeding of these rotifers, parthenogenetic propagation produces up to 200 grams of rotifers per cubic meter of culture. If you need a freshwater rotifer, breeding begins at least a week in advance. Cultivation is carried out as follows.
Since the seventies of the last century, aquarists have used a unique apparatus for cultivating rotifers. The cultivation vessel itself is a container with a height exceeding 25 centimeters with a glass bottom. Illuminators are placed on top of the vessel and under it. At the initial stages of cultivation, lighting is produced from below. Since rotifers have positive phototaxis, that is, they move towards the light, they lower down and feed on bacteria that multiply on the remains of algae. At the same time, the light that passes through the sludge promotes photosynthesis, which saturates the water with oxygen. As the culture of rotifers develops and they multiply en masse, the amount of oxygen dissolved in the water decreases, and they begin to rush to the surface. This point indicates the need to remove from the vessel some of the rotifers that are used to feed the fry. To increase the number of such rotifers, the light supplied from below is turned off and the overhead lighting is turned on. The rotifers gathered at the surface are collected using a siphon and fed to the fish, and, if necessary, new fresh phytoplankton is added to the cultivator vessel.
Thanks to the strong overhead light, phytoplankton reproduces, saturates the water with oxygen, and the rotifers that remain in the cultivator are distributed in the water column. At this moment, the upper light is turned off, the lower one is turned on, and the process of intensive breeding of rotifers is repeated.
To obtain resting eggs for subsequent reproduction, it is enough to lower the temperature, simulating the onset of winter. The maximum egg yield is obtained when the cultivator population density is 120-150 eggs per cubic centimeter of culture.
Freshwater rotifer Philodina acuticornisodiosa
Photo 11 Rotifer Philodina acuticornisodiosa
This rotifer is somewhat larger than the rotifers of the genus Brachionus.
The body shape of phylodines is similar to an elongated cone, at the front end of which there is a rotary apparatus.
Photo 12 Rotator apparatus of phylodyne.
The rear end, called the leg, is pointed and has two finger-like projections called the “grasping fork.”
Photo 13 Grasping fork
With the help of a rotating apparatus, phylodines move in the water column, and using their legs they move along the substrate.
Philodines usually live at the bottom of aquariums among silt particles. Their size makes these neighboring fish quite visible under a strong magnifying glass. They can be seen if you carefully examine the bottom silt, how these tiny animals crawl along the substrate. The movement occurs as follows. Philodina attaches itself to the substrate with the help of its leg.
Photo 14
Photo 15 The arrow marks the place of attachment to the substrate
Photo 16 Then stretched out
And it is attached to the substrate by the anterior end of the body. The arrow marks the place of attachment
Photo 17
Then it lifts the back of the body from the substrate and quickly pulls the leg towards the head.
Photo 18
And it attaches its foot to the substrate again and begins to prepare for a new “step”.
Photo 19
The second method of movement of phylodines is slow swimming with the help of synchronous vibrations of the cilia of the rotator apparatus.
Philodine is cultivated in the same cultivators as other rotifers. There are differences regarding the nutrient substrate. The average lifespan of phylodines is 27 days. Cultivation temperature is in the range of 24°C - 27°C. Yeast is not introduced - on the contrary, it inhibits the growth of these animals. The main food is bacteria and algae. The most commonly used bacteria are Bacillus subtilis and lactic acid bacteria.
The nutrient solution is prepared as follows. 10 grams of hay in a liter of distilled water are brought to a boil, infused for three days, filtered and two liters of distilled water are added per liter of infusion. To maintain the culture, add one or two drops of milk (boiled) 2-3 times a month. Philodine can be cultivated together with slipper ciliates on dry banana peels with the addition of milk to the culture.
Hungry phylodines, as a rule, sink to the bottom and move along the substrate on particles of silt.
Well-fed rotifers constantly swim in the water column.
Photo 20
Philodines grow well and multiply vigorously on a substrate such as the excrement of fish larvae. Excellent substrate and remains of maltwater rotifers that died in fresh water. Aquarists use this feature of this type of rotifer to clean the aquarium in which the fry are kept, combining feeding and cleaning.
A sharp increase in the growth rate of rotifers can be achieved by introducing bacteria that live above the surface of activated sludge into the culture.
Good results are also obtained when rotifers feed on algae such as chlorella or scenedesmus. It is necessary to adhere to optimal concentrations, for example, for chlorella it is 0.4 grams per liter, with a deviation either downward to 0.1 grams per liter or up to a twofold excess, leading to a sharp decrease in the survival rate of rotifers.
For successful cultivation it is important to provide sufficient oxygen. With a lack of O₂ and weak blowing, rotifers of this species begin to accumulate at the water/air interface, forming dense clusters on the walls of the cultivator.
The disadvantages of feeding with phylodines include the ability of these rotifers to burrow into the mud in search of food, which makes them inaccessible to the fry. Therefore, when feeding young fish with phylodines, you need to make sure that they are full, since in this state they prefer to stay in the water column, and make sure that silt does not accumulate at the bottom.
Photo 21 Asplakhna under a strong magnifying glass
Freshwater rotifer Asplanchna priodonta
Asplanchna priodonta, which aquarists simply call asplanchna, is the largest rotifer and is used to feed fry. It reaches one and a half millimeters, is constantly located in the water column, being a typical planktonic organism, so it is given not only to fry, but also to adult fish, for example neon, small species of characins. It is a predator that eats mainly ciliates, as well as smaller rotifers.
The asplachna moves forward with its mouth end, without rotating around an axis, like other rotifers, it is constantly in motion. Therefore, this is not a very suitable type of food for slow-moving fry. The body of the rotifer is round, there is no leg.
Asplachn is grown in accordance with its food preferences - ciliates and rotifers of the genus Brachionus are used as food. Those who require this rotifer cultivate on malt-water species as food. Culture Br. Plicatilis are desalinated as described above, and then the Asplanchna priodonta culture is introduced.
Typically, feeding with asplachnas is used during periods of transition of fry from smaller feeds to larger types of feed.
Photo 22 Asplanchna priodonta, modern scanning microscopy
The nutritional value of Asplanchna priodonta is one of the lowest among rotifers, therefore, before giving them to fish, the rotifers themselves are intensively fed.
Equipment for rotifer cultivation
Those who are interested in how to breed rotifers and cultivate them at home can find a description of many cultivators for these organisms in the book “Plankton Culture Manual” by Frank H. Hoff and Terry W. Snell. Despite all the differences, all cultivators are united by one principle - the principle of the device, which can be seen in the photo of one of the systems for cultivating rotifers.
Photo 23
Fresh water bottles for coolers and plastic 2-liter bottles for mineral water or refreshing drinks are used as containers. Hoses are silicone.
Many aquarists, becoming more and more interested in their hobby, come to the desire to try to breed their pets, which means they need starter food. This has prompted the supply of various industrially manufactured cultivators. For those who are interested in rotifers, breeding these organisms at home offers various options.
These are simple ones, similar to the one shown in the photo, and complex complexes.
In the 90s, work began in Japan on the creation of a new type of cultivator (Yoshimura, 1995, Fu, 1997), which in the 21st century culminated in the creation of a new type of cultivator. These are complex systems in which the cultivation space is divided into three separate zones:
— cultivation zone;
— filtration zone;
- collection area.
A medium containing food (for example, the most commonly used Chlorella vulgaris) is continuously supplied to the cultivation zones and the same amount of water is at the same time filtered out into the filtration zone, in which fillers with nitrifying bacteria are placed, undergoes biological purification, and is enriched with nutritional organisms for rotifers. and returns to the cultivation area. When the required density of rotifers per established unit of volume is reached (set automatically based on changes in the light transmittance of the culture (optical density) in the cultivation zone), they are removed to the collection zone from where the rotifers are filtered and given to the fry.
Collection area
At this stage, the culture medium passes through special filters that prevent organic waste from entering the feed and contaminating the collection net used when catching rotifers.
Closed water supply cultivators have appeared with the installation of a flotator and an ozone generator installed in the water movement cycle in the area before the submersible biofilter charged with nitrophizing bacteria.
Photo 24 Diagram of a modern cultivator for breeding rotifers, controlled by a computer.
Modern industrial installations for the cultivation of Brachionius plicatilis using modern food additives make it possible to obtain cultures with a density of 23,000 individuals per milliliter. At the same time, the levels of ammonia and nitrites are quite low:
— for ammonia 0-0.8 ml/l;
— for nitrites 0.2 – 3.5 milligrams per liter.
The pH level is also stably maintained at 7.3, which is achieved by introducing carbonate plates into the filter design.
Read more about feeding fry in the articles:
“Nematode microworms - food for fry in the aquarium”
“Standard requirements to be observed when feeding fry”
“Successful rearing of fry = algorithm for proper feeding, conditions of detention”
“Ciliates for fry - breeding at home (where to get them, how to breed - breed - grow) and feeding (how to feed)"
“Artemia salina is a universal food: eggs, larvae, mature crustaceans for fry, juveniles and adult freshwater and marine fish, as well as shrimp, corals and other aquatic organisms”
“Dietary supplements (probiotics, feed additives) for aquarium fish – an important step in the development of modern aquarium farming”
Life cycle features
Considering the defenselessness of the Rotifer, nature took care of the safety of the species, rewarding it with an unusual reproduction cycle.
Under favorable conditions, females reproduce females without the participation of males. In cases of danger (drying out of the reservoir, severe frosts), male individuals appear and take part in the process. As a result, females lay eggs enclosed in a dense cocoon and wait in this state for improved conditions. Subsequently, females hatch from them and are capable of self-reproduction, and parthenogenesis is repeated until the next stress.
There are classes that survive for millions of years without sexual reproduction of their own kind. Despite this, new species appear and the factor of variability continues to work. Scientists suggest that the worms are capable of using the genes of alternative living creatures that are similar in size.
Links[edit]
- ^ ab Howey, Richard L. (1999). "Welcome to the wonderfully strange world of rotifers". Micscape Magazine. Retrieved February 19, 2010.
- ^ ab Harmer, Sidney Frederick and Shipley, Arthur Everett (1896). Cambridge Natural History. Macmillan Company. P. 197. Retrieved July 25, 2008. John Harris Rotifer.
- "Rotifers". Freshwater life
. Archived from the original on August 1, 2012. Retrieved February 19, 2010. - Hendrik Segers (2007). An annotated checklist of rotifers (Phylum Rotifera) with notes on nomenclature and taxonomy
- Gomez, Africa; Serra, Manuel; Carvalho, Gary R.; Lunt, David H. (July 2002). "Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera)". Evolution; International Journal of Organic Evolution
.
56
(7):1431–1444. DOI: 10.1111/j.0014-3820.2002.tb01455.x. ISSN 0014-3820. PMID 12206243. - ↑
Dec 2011 4th Internat. Barcode of Life Conference, University of Adelaide - ^ ab Bourne, A.G. (1907). Baines, Spencer and W. Robertson Smith (eds.). Encyclopedia Britannica. XXI
(Ninth ed.). Akron, OH: Werner Company. clause 8. - Barnes, R. S. K.; Calow, P.; Olive, P. J. W.; Golding, D. W. and Spicer, D. I. (2001), Invertebrates: a synthesis
, Oxford; Malden, MA: Blackwell, ISBN 978-0-632-04761-1, par. 98 - Bakai, Aisha; Guruswamy, Vivek; Liu, Janie and Rizky, Gizem (May 1, 2000). "Introducing Rotifers". University of California Museum of Paleontology. Retrieved July 27, 2008.
- Shimek, Ronald (January 2006). “Nano-animals. Part I: Rotifers". Reefkeeping.com. Retrieved July 27, 2008.
- Ruppert, Edward E.; Fox, Richard S. and Barnes, Robert D. (2004), Invertebrate Zoology: A Functional Evolutionary Approach (7th ed.), Belmont, CA: Thomson-Brooks/Cole, ISBN 978-0-03-025982-1, item 788ff. - see in particular page 804
- Pieczenik, Jan A. (2005). Biology of Invertebrates
. Boston: McGraw-Hill, Higher Education. p. 178. ISBN 978-0-07-234899-6. - ^ abcdefghijkl Barnes, Robert D. (1982). Invertebrate Zoology
. Philadelphia, PA: Holt-Saunders International. pp. 272–286. ISBN 978-0-03-056747-6. - ^ a b Robert Lee Wallace. "Rotifers: exquisite metazoans". 2002. doi:10.1093/icb/42.3.660 citation: "What is the function of the retrocerebral organ?"
- ↑
Jessica L. Mark Welch, David B. Mark Welch, and Matthew Meselson (February 10, 2004).
"Cytogenetic evidence for asexual evolution of bdelloid rotifers". Proceedings of the National Academy of Sciences
.
101
(6):1618–1621. Bibcode: 2004PNAS..101.1618W. DOI: 10.1073/pnas.0307677100. PMC 341792. PMID 14747655. - ^ abc Nogradi, T., Wallace, R.L., Snell, T.W., 1993. Rotifera vol.1: biology, ecology and systematics. Guides to the identification of microinvertebrates from the world's continental waters 4. SPB Academic Publishing bv, The Hague.
- Klaus-Peter Stelzer, Johanna Schmidt, Anneliese Wiedlroither & Simone Riess (20 September 2010). "Loss of sexual reproduction and dwarfism in small metazoans". PLoS ONE
.
5
(9):e12854. Bibcode: 2010PLoSO...512854S. DOI: 10.1371/journal.pone.0012854. PMC 2942836. PMID 20862222. CS1 maint: multiple names: list of authors (link) - Wurdak, Elizabeth S.; Gilbert, John J.; Jagels, Richard (January 1978). "Fine structure of resting eggs of the rotifers Brachionus calyciflorus and Asplanchna sieboldi". Proceedings of the American Microscopic Society
.
97
(1):49–72. DOI: 10.2307/3225684. JSTOR 3225684. PMID 564567. - Clément, P.; Wurdak, E. (1991). "Rotifera". In Harrison, F.W.; Ruppert, E. E. (ed.). Microscopic anatomy of invertebrates
. Ashelminthes, vol. IV. Wiley-Liss. pp. 219–97. - Marcus, Nancy H.; Lutz, Robert; Burnett, William; Cable, Peter (January 1994). "Age, viability and vertical distribution of zooplankton resting eggs from an anoxic pool: evidence from an egg bank". Limnology and Oceanography
.
39
(1): 154–158. DOI: 10.4319/lo.1994.39.1.0154. - Kotani, T.; Ozaki, M.; Matsuoka, K.; Snell, T. W.; Hagiwara, A. (2001). "Reproductive isolation among geographically and temporally isolated marine strains of Brachionus". Rotifer IX
. pp. 283–290. DOI: 10.1007/978-94-010-0756-6_37. ISBN 978-94-010-3820-1. - Garcia-Roger, Eduardo M.; Carmona, Maria Jose; Serra, Manuel (January 2005). "The nature of degradation of diapausing eggs of the rotifer species Brachionus (Müller, 1786)". Journal of Experimental Marine Biology and Ecology
.
314
(2):149–161. DOI: 10.1016/j.jembe.2004.08.023. - ^ ab Hespeels B, Knapen M, Hanot-Mambres D, Heuskin AC, Pineux F, LUCAS S, Koszul R, Van Doninck K (July 2014). “The path to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga subjected to drying" (PDF). J. Evol. Biol
.
27
(7): 1334–45. DOI: 10.1111/jeb.12326. PMID 25105197. - Wallace, R. L., T. W. Snell, S. Ricci, and T. Nogrady (2006). Rotifera Vol. 1: Biology, ecology and systematics. Guides to the identification of microinvertebrates of the world's continental waters 23
, 299 pp. Kenobi, Ghent / Backhuys, Leiden - Fleet, Jean-François; Gespils, Boris; Li, Xiang; Noel, Benjamin; Arkhipova Irina; Danchin, Etienne GJ; Heinol, Andreas; Henrissat, Bernard; Koszul, Romain; Oury, Jean-Marc; Barbe, Valerie; Barthelemy, Roxanne-Marie; Bast, Jens; Bazykin, Georgy A.; Chabrol, Olivier; Kulu, Arnaud; Da Rocha, Martina; Da Silva, Corinne; Gladyshev, Evgeniy; Gure, Philip; Hallacek, Oscar; Hecox-Lee, Betty; Labadie, Karin; Lejeune, Benjamin; Piskurek, Oliver; Poulain, Julie; Rodriguez, Fernando; Ryan, Joseph F.; Vakhrusheva Olga A.; Weinberg, Eric; Wirth, Benedict; Yushenova Irina; Kellis, Manolis; Kondrashov, Alexey S.; Mark Welch, David B.; Pontarotti, Pierre; Weissenbach, Jean; Winker, Patrick; Jayon, Olivier; Van Doninck, Karin (21 July 2013). "Genomic evidence for ameiotic evolution of the bdelloid rotifer Adineta vaga". Nature
.
500
(7463): 453–457. Bibcode: 2013Natur.500..453F. DOI: 10.1038/nature12326. PMID 23873043. - Stelzer, C. P. (2011). First assessment of genome size diversity in monogononous rotifers. Hydrobiologia 662: 77–82 https://doi.org/10.1007%2Fs10750-010-0487-1
- Stelzer, C.P., Riss, S., Stadler, P. (2011) Evolution of genome size at the speciation level: the cryptic species complex Brachionus plicatilis
(Rotifera) BMC Evolutionary Biology 11: 90.
Feeding and rearing
Depending on the species, Rotifers are fed using different methods. Let's consider the diet of the most popular and valuable worm.
At home, Rotatoria is fed with yeast cultures; you can use baking or hydrolysis products. 2.5 grams of mushrooms are diluted in water (10 liters) and poured into a container where the worms live. Cloudiness of the liquid occurs. As soon as transparency returns, add a new portion. Half of the solution is replaced every month.
Regardless of the conditions of detention and care, the worm colony gradually degenerates, but this is not scary. Eggs remain at the bottom of the container, with the help of which the appearance can be easily restored. First, filter the bottom sediment through thin paper or a napkin. The resulting material is dried in a dark, well-ventilated area, then transferred to the refrigerator for storage. When the colony needs renewal, the eggs are added to a fresh saline solution. For accelerated incubation, it is recommended to keep the culture for several months at a temperature of -5...-10 °C.
Rotatoria is a salt-water species and can die at low salt concentrations in water. When the chemical composition of the liquid changes, the worms sink to the bottom and die within an hour, which is how food is used for fry of bottom-dwelling fish species. For pets living and feeding in the middle layers, Rotifer is prepared gradually, reducing the salt concentration to 3 grams per liter of water. The worm lives in this solution for up to 3 days, but it is advisable to slightly aerate the tank.
Preparing to feed the fry requires some special equipment. Use a net with a gas cloth for filtering. It is impossible to remove the fabric from the solution, since the worm quickly dies and sticks together without water. Carefully and quickly transfer the net to the aquarium.
Reproduction and breeding
Certain types of rotifers are suitable for home breeding. For example, Philodina requires a special environment to reproduce. Hay is boiled in distilled water at a rate of 10 g/l, cooled and left to infuse for 48-72 hours. Then the solution is passed through a filter and diluted with distilled water in a ratio of 2:1. Liquid with rotifers is poured into it and maintained by adding 1-2 drops of boiled milk to the container 2-3 times a month. With weak aeration, accumulations of Philodina will appear on the walls of the tank closer to the surface of the water.
To breed freshwater rotifers Brachius Caliciflorus, “blooming” water from an aquarium with numerous floating microscopic algae, or baker’s or hydrolysis yeast (0.02 g/l) is added to the culture container. Some aquarists grow them in a pale green infusion of nettle leaves mashed and scalded with boiling water at a temperature of 25-30 0C.
Economic importance
Rotifers are preyed upon by many animals, such as copepods, fish (eg, herring, salmon), gastropods, comb jellies, jellyfish, starfish, and tardigrades.
About 1,500 species of rotifers are known, in Russia there are about 600 species.
Systematics of the Rotifera type:
- Class: Eurotatoria De Ridder, 1957 = Eurotatoria Subclass: Bdelloidea Hudson, 1884 = Leech-like rotifers Order/Order: Bdelloida Hudson, 1884 = Family: Adinetidae =
- Family: Habrotrochidae =
- Family: Philodinavidae =
- Family: Philodinidae =
- Superorder/Superorder: Gnesiotrocha Kutikova, 1970 = Order/Order: Collothecaceae Harring, 1913 =
- Squad/Order: Ploima Hudson and Gosse, 1886 =
- Subclass: Seisonacea Wesenberg-Lund, 1899 = Family: Seisonidae Wesenberg-Lund, 1899 =
References: [1] A. Dogel. Zoology of invertebrates. Edition 7, revised and expanded. Moscow "Higher School", 1981 [2] Zoology course. B. A. Kuznetsov, A. Z. Chernov, L. N. Katonova. Moscow, 1989 [3] Key to freshwater vertebrates of the European part of the USSR (plankton and benthos). Gidrometeoizdat. Leningrad, 1977
They are alive!
In an email written to Live Science, Stas Malavin, a researcher at the Institute of Physicochemical and Biological Problems of Soil Science, located in the city of Pushchino, Moscow region, spoke in more detail about the experiment:
“We placed a sample of soil extracted from permafrost into a Petri dish filled with a nutrient solution. And they waited a little. And our patience was rewarded! Frozen organisms are released from their dormant state. And they began to actively move and reproduce!”
The new rotifers were so genetically identical to the old ones that the researchers couldn't tell them apart.
The fact that a complex living being was successfully resuscitated from a state of almost complete metabolic arrest (or cryptobiosis) is simply amazing. But in reality, this is probably quite normal for rotifers. Because it was not without reason that they evolved in such a way as to be able to use cryptobiosis at periods convenient for them. After all, the rotifer lives in water. And it can dry out at any moment. Or freeze. In addition, the rotifer can repair its DNA, which may be damaged after a long sleep. And it is able to protect its cells from molecules that damage them.
Habitat
Most rotifers are free-living organisms (some are parasitic in organisms of various classes of chordates). Rotifers live mainly in aquatic environments. Most worms live in fresh water bodies, but there are classes and species that live in the seas. Some representatives have the ability to remain in a state of cryptobiosis for a long time when reaching land due to the tidal cycle. This adaptation makes the worms invulnerable to the tidal factors of the environment.
Sleep and not grow old
In science fiction movies, you might have seen devices that allow a person to fall into suspended animation for many years. Ellen Ripley, the heroine of films about scary xenomorphs, spent, for example, 57 years in an artificial hibernation module! And at the same time she aged a maximum of 5 years. Do you remember there was also a film where a selfish man woke up a beautiful woman who was in a state of suspended animation. Than doomed her to life until her death on board a spaceship flying towards a distant star at great speed.
But this is all just a slowdown in metabolism and sleep. Unnaturally long and artificial. But a dream. This state cannot last, for example, a couple of thousand years. Because the body still spends its resources little by little. But if you freeze a person, then it’s a different matter! Then his body can be stored almost forever! It will be able to calmly cross distances of hundreds of light years in this form.
A rotifer will not recognize its native swamp after 24,000 years. Photo by NASA.
However, freezing is half the battle. How can this frozen organism be brought back to life? That is the question. But perhaps we are taking the first steps towards solving this problem.