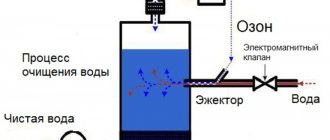
There are quite a few types of liquid filtration systems. Each of them operates according to its own parameters and technological features. Current water treatment equipment manufacturing companies regularly offer customers the latest developments in this area. Today it’s worth talking about biofilters for wastewater treatment, what they are made of and how they work.
Why do you need a biofilter?
Biological filter systems purify the air or water environment from organic compounds present in them. These units do an excellent job of eliminating organics, dust particles, unpleasant odors, formaldehyde and phenolic components, aerosol vapors and acidic components.
Such devices are used at wastewater treatment stations, solid waste disposal sites, waste treatment plants, chemical plants (manufacturing paint and varnish products, painting materials, etc.). Such devices are no less widely used in agriculture and the food production and processing industry.
Cleaning technology
Sewage from the house through a pipe enters the first compartment of the septic tank, where it is separated into heavy and light particles. The heavy ones settle to the bottom, and the light ones flow into the second compartment (there can be more than two such compartments). Here, organic matter begins to be processed by aerobic putrefactive bacteria, during which carbon dioxide and silt deposits are released. The latter settle to the bottom. Such septic tanks guarantee that the output water will be 90% pure. So, a tenth will still be dirty, so a biofilter comes into play.
It is very important to understand that purely structurally this device can be different. For example, this can be a compartment, as an integral part of the septic tank itself, or as a separate tank. The purified water can pass from above, going down, or enter from below, passing through the filter material and being removed along the upper level.
Classification
When understanding the question: what is a biofilter, it is necessary to find out what materials are used in the filtration process. Based on these parameters, devices are divided into several types.
With volume load
They are divided into a number of subtypes:
- Drip type, characterized by low productivity. The grain size of the loaded object is 20-30 mm with a layer height of 2 m.
- Highly loaded. The size of the material to be immersed is 40-60 mm and a four-meter layer surface.
- Tower. They reach 16 meters in height, the grain size is 40-60 millimeters.
Flat loading
The links of the classification line look like this:
- Increased rigidity is provided by concrete rings, pipe elements, etc. Metal, ceramics or plastic divided into small parts is placed in a container of a certain volume. The laying density must be at least 600 kg per cubic meter. The permissible porosity is at least 70%. The filter base reaches 6 m.
- These wastewater filters are characterized by a rigid load of blocks or grids. Block elements are made from asbestos with a density of up to 250 kg per cubic meter and a porosity of 80% per 6 meters of filtrate. In addition, plastic materials are excellent; due to their characteristics, the filter layer can be about 16 m.
- Rolled (aka soft) is created using durable metal mesh, synthetic fabrics or plastic coating. The weight is placed in the form of rolls or secured in a special way to the frame. Density parameters - 60 kg/m³, porosity almost 100%, height 8 m.
- Devices for placement in containers with a curved bottom. Plastic or asbestos discs are placed on top of the liquid to be cleaned. They are located at a distance of 10-20 mm from each other. The size varies from 0.6 to 3 meters in diameter.
Roll and fill methods are used for the highest flow rates up to 10,000 m³/day. Hard with the use of blocks with an average of 50,000 cubic meters per day. Submersible versions are only relevant for light loads.
Drip
MBFT-75 Membrane for 75GPD
SF-mix Clack up to 0.8 m3/h
SF-mix Runxin up to 0.8 m3/h
Such biofilters for water purification are a fundamentally different filtration technology. The aqueous solution is supplied by spraying drops or jet method. In this case, oxygen is carried through the drainage base of the filter unit or supplied from the surface.
The pre-filtered waste liquid with a small amount of polluting particles enters the distribution block, which redirects it to the loading layer. After this, the liquid medium is sent to the drainage and water tanks outside the territory of the bioremediation system. The biofilm is separated in the secondary settling tank.
These systems are characterized by a reduced organic load. To clear the filter material from the non-living shell, hydraulics are used.
During the cleaning process, uniform dispersion of the complete contents of the biofilter must be ensured. Otherwise, a load surge cannot be avoided. Drip-based devices are almost impossible to control by changing external conditions. When using them, the levels of contamination and the condition of the units themselves are monitored. The cleaning process is quite expensive, so it is easier to completely change the loaded material.
When using this biological water filter, carefully monitor aeration. The oxygen level should not drop below 2 mg/l. In this case, it is necessary to simultaneously clean the area under the drainage and above the bottom of the container. The bio filter based on drip action is very sensitive to strong winds in winter. Therefore, for maximum effective functioning, wind protection is organized. In addition, stable operation is disrupted by waterlogging of the filter base, which must be urgently eliminated and foreign objects in the total loading mass.
Highly loaded
The operation of this type of biofilter is characterized by high air exchange and, as a result, excellent oxidation ability. A large amount of incoming air is made possible due to the large loading fraction and increased water load.
The filtered liquid moves quite quickly and carries with it non-oxidizable elements, as well as parts of the failed biofilm. All available oxygen is withdrawn to eliminate remaining contaminants.
Bio filters of this type are distinguished by the height of the loaded layer, the large grain size of the drainage base and the special structure of the bottom of the tank, which ensures better air circulation. Cleaning of such a device is possible only with a continuous pressure supply of liquid.
What does household wastewater consist of?
Modern houses must have plumbing and sewer systems. People use the first for drinking and household needs, and with the help of the latter they remove contaminated liquid. And although the level of impurities in wastewater does not exceed 1%, it cannot be reused until it has been treated.
The composition of wastewater varies significantly depending on the location and time of sampling. Typically, a liquid contains both solid and soluble elements. Inorganic substances can be eliminated with a simple filter, but organics require a more complex approach. If measures are not taken, there is a risk of decomposition of biological compounds, which leads to the formation of a putrid odor and spoilage of water.
Effluents may contain residues of fats, detergents, phosphates and chloride compounds, nitrogen, sulfate and petrochemical products. These substances do not disappear anywhere on their own, so biological wastewater treatment is used to remove them.
Particular attention should be paid to this issue in country houses that are not connected to a centralized water supply. A well and a cesspool are usually located on their sites. Under unfavorable circumstances, untreated wastewater may end up in the tap, which will lead to the risk of poisoning of all residents.
Wastewater treatment Source vagner-ural.ru
Composition and principle of operation of the biofilter
The components of the filter unit are:
- The base of the treatment plant is a cleaning load located in a container (a kind of aquarium), into which access to an aqueous solution is organized. Materials: plastic, crushed stone, slag elements, expanded clay stone, etc. must have low density and large size.
- Mechanism for redirecting fluid. It makes it possible to uniformly irrigate the filter base contaminated with a liquid medium.
- Drainage layer.
- A device that distributes oxygen throughout the system. It supplies air to carry out oxidation reactions.
Now you should understand how contaminated water is purified in biofilters. Oxidation processes in bio filters are quite similar to spraying fields or water treatment in biotreatment systems. They just differ in greater intensity.
The loading matter filters out insoluble trace elements from H2O remaining after a series of settling tanks. Thanks to the biofilm, organic impurities are adsorbed. Living organisms live in this film due to the decomposition of organic matter. In addition, part of it goes to the growth of the total biomass of the layer. Thus, two useful actions can be observed simultaneously:
- removing excess “life” from the liquid;
- biofilm growth.
Along with wastewater flows (including from septic tanks), dead shell elements disappear. Air circulation is ensured by an established ventilation system.
Biofilter diagram
SF-mix manual up to 0.8 m3/h
AMETHYST - 02 M up to 2 cubic meters/day.
Aeration unit AS-1054 VO-90
The schematic image of the filter structure located below will help to visualize the functioning process.
Calculation
Its implementation for drip structures is advisable in relation to determining the optimal thickness of the loading layer, its weight, establishing the parameters of water distribution devices, drainage systems and drainage tanks.
The preferred size of the loading volume is calculated relative to the oxidation power - OM. This value represents the mass of air required per day. This element is influenced by temperature conditions, materials used, type of pollutants, air exchange method, etc. When the average annual temperature stably does not exceed 3°C, the treatment structure is moved to a warmer place (artificially heated). In this case, it is necessary to provide a fivefold supply of oxygen.
To organize the process, perform the following steps:
- calculate the coefficient K;
- determine the height and maximum possible hydraulic load;
- calculate the total area.
For highly loaded filters, a different technique is used:
- First, the concentration of pollutants is determined;
- then proceed to calculations of the recirculation coefficient;
- Finally, they deal with the expected size of the filter base.
In addition to the above, there are other methods for calculating biofilters that are no less accurate. But their implementation involves the use of more complex forms.
Calculation stages
For ease of perception, they should be presented in the form of a short list:
- Determination of the structure of polluting elements. This will allow you to select the most suitable type of living organisms to combat them.
- Calculating the size of the reservoir to ensure even distribution of beneficial bacteria inside.
- Calculation of the optimal speed of the treatment process. This must be done taking into account the vital activity of microorganisms. They should have time to eliminate extraneous components.
- Development of dimensional drawing.
How filtering works
Once again let's return to the question: how does purification occur in biofilters? The functioning of such systems is based on the natural ability of microorganisms to capture organic and inorganic matter from water flows passing through them. They can also affect organic compounds of artificial origin, initiating the process of decomposition and oxidation. As a result, only liquid and carbon dioxide remain.
The main cleaning element is the filter layer. On its surface, living organisms create a biologically active shell. Contaminant components pass through it and are sorbed. At a further stage, they (already in a dissolved state) attach to bacterial cells and disintegrate completely.
The materials for the cleansing base are natural substances.
The air filter is capable of operating in humid environments from 60 to 100% humidity.
Main table dispenser AquaPro 919H/RO (hot and cold water)
Main table dispenser AquaPro 929CH/RO (cooling/heating)
Floor dispenser AquaPro 311 (empty, without cooling)
Processes taking place in a biological filter for an artificial reservoir.
Nitrification is a two-step process of converting ammonium (NH+4) into nitrites (NO-2), and nitrites into nitrates (NO-3). The first stage of this process (the conversion of ammonium to nitrites) is carried out by autotrophic bacteria of the genus Nitrosomonas, which, in the presence of oxygen, are able to oxidize ammonium to nitrites, using approximately 270 kJ/mol as an energy source.
In the second stage of the nitrification process, approximately 73 kJ/mol is released during the oxidation of nitrites to nitrates. This reaction is carried out by bacteria of the genus Nitrobacter, which use the released energy for metabolic processes.
The reactions of converting ammonium into nitrite, and nitrite into nitrate, require a significant amount of oxygen. The stoichiometric oxygen requirement for the conversion of ammonium into nitrite is 3.43 kg of oxygen per 1 kg of oxidized ammonium. The conversion of nitrite to nitrate requires 1.14 kg of oxygen per 1 kg of oxidized nitrite. Thus, the conversion of 1 kg of ammonium to nitrate requires 4.57 kg of oxygen. Since the operation of a biological filter requires at least a stoichiometric amount of oxygen, the supply of oxygen can become a factor limiting the nitrification processes occurring in the biofilter.
The chemical processes of ammonium-nitrite-nitrate conversion are used by Nifrosomonas and Nitrobacter bacteria to produce energy. Bacteria utilize this energy, carbon dioxide and oxygen to produce organic compounds necessary for cell growth and metabolism. The volume of cell mass formed by growing bacteria is of great importance for biological filters, since dying bacteria form particles suspended in water that clog the filter, which leads to the formation of anaerobic areas in it, large pressure losses of water flowing through the filter and other undesirable consequences.
When 1 kg of ammonium is oxidized to nitrate, 147 g of Nitrosomonas cells and 20 g of Nitrobacter cells are formed. Fortunately, compared to heterotrophic bacteria, nitrifying bacteria produce a small mass of cells per mass of oxidized substrate, and as a result, filters do not clog as much if the amount of organic matter entering the nitrification filter is kept to a minimum. Carbon dioxide (CO2) is involved in the synthesis reaction of cells of autotrophic organisms. Carbon dioxide partially meets the need for oxygen, being also a source of carbon.
The bacterium Nitrosomonas consumes 3.02 kg of oxygen for every kilogram of ammonium oxidized to nitrite, and Nitrobacter consumes 1.02 kg of oxygen per kilogram of nitrite oxidized to nitrate. Thus, the total oxygen consumption is 4.04 kg per kilogram of ammonium oxidized to nitrate. However, since this ratio depends on the age of the crop, the amount of oxygen required to completely oxidize 1 kg of ammonium to nitrate ranges from 4 to 4.6 kg.
Rice. 2. Nitrification curves.
In Fig. Figure 2 shows the relationship between the content of ammonia, nitrites and nitrates at the initial stage of operation of the biological filter. At the beginning of the operation, the population of nitrifying bacteria is either small or absent, so adding fish to the system will quickly increase the concentration of ammonia in the water. This point is critical, since even low concentrations of ammonia can be detrimental to cultured organisms. A decrease in ammonia concentration usually signals the emergence of a resilient population of Nitrosomonas, which converts ammonia into nitrites. However, such an assumption can lead to dangerous complacency. Typically, a biological filter contains both heterotrophic and autotrophic (nitrifying) bacteria. Heterotrophic bacteria are characterized by a more accelerated generation time (i.e., time to grow, reproduce, and produce a large population) than autotrophic bacteria—therefore, heterotrophic bacteria, which use a source of simple organic carbon but require inorganic nitrogen for metabolism, initially become dominant in the filter and utilize the ammonia produced in the system. A decrease in organic carbon content suppresses the growth of heterotrophic bacteria. Therefore, an initial decrease in ammonia concentration may not indicate nitrification, but rather the presence of a large population of heterotrophic bacteria. Fortunately, this is not often observed in aquaculture.
An increase in the Nitrosomonas population leads to an increase in nitrite concentrations, which reaches a maximum and then begins to decline as the Nitrobacter population grows, converting nitrites to nitrates. Increased concentrations of nitrites are also dangerous because they are toxic to fish and many other aquatic organisms. Systematic monitoring of nitrite and ammonia concentrations makes it possible to ensure that nitrification is taking place.
In any aqueous system, ammonia is present in the form of NH+4 and NH3, since there is an equilibrium between these two forms in water. The nonionic form of ammonia (NH3) is toxic to most aquatic organisms at concentrations of 1 mg/L and below. Salmon are particularly sensitive to NH3 and are adversely affected by prolonged exposure to water with an NH3 concentration of 0.006 mg/l. The equilibrium between the NH3 and NH+4 forms depends on pH (Fig. 3).
Rice. 3. The influence of pH and temperature on the distribution of ammonia and ammonium ions in water
At a pH less than 7, the concentration of NH3 is low and the risk of toxicity is reduced. At higher pH values, the toxicity of NH3 increases. It should be noted that saltwater systems typically have a pH of 7.5 to 8.3, while freshwater systems typically have a pH of 6.5 to 7.8.
Nitrification and bacterial synthesis produce hydrogen ions, so pH tends to decrease during nitrification. In aqueous solutions, hydrogen ions are neutralized by bicarbonate ions (C02) in water (at pH less than 8.5).
H+ + HCO-3 → CO2 + H2O
The end result of this process is a decrease in bicarbonate concentration and an increase in carbon dioxide, both of which contribute to a decrease in pH. Approximately 7.13 kg of bicarbonate in the form of CaCO3 is used to neutralize hydrogen ions formed during the oxidation of 1 kg of ammonium. Calculations taking into account the equilibrium of carbonic acid in fresh water show that if all the carbon dioxide formed during neutralization remains in solution, and the pH should be above 6.0, then the amount of ammonium (in mg) per liter that can be oxidized is approximately 0.1 alkalinity, expressed in CaCO3. However, in most closed systems, partial removal of carbon dioxide occurs (for example, through aeration) and there is usually some reduction in pH, so the 0.1 alkalinity rule provides a conservative estimate. However, carbonate or bicarbonate usually needs to be added to water with low alkalinity to maintain pH at the proper level. This is often accomplished by using carbonate rocks as a filter layer.
The pH of the system also has a direct effect on nitrifying bacteria. There are different opinions on the effect of pH on the rate of oxidation by nitrifying bacteria. In some experiments, it was observed that the rate of ammonium oxidation by unadapted cultures is constant only at pH 6.8-8. Below pH 6.8, the rate of ammonium oxidation decreases rapidly with decreasing pH. No experiments were carried out for pH above 8. In other experiments, a constant rate of oxidation was observed for Nitrosomonas europaea in the pH range from 7.5 to 9, and for Nitrosomonas bacteria, only in the range from 8.5 to 8.8. All data, with the exception of the last one, indicate that Nitrosomonas bacteria appear to have the highest and relatively constant rate of oxidation at pH levels between 7 and 9. However, over time, filters can acclimate to lower pH values. Experiments showed that 10 days after the pH was reduced to 6, the bacterial rate of ammonium oxidation to nitrite was equal to the rate observed at the optimal pH range. However, lowering the pH to 5.5 created conditions to which the nitrifying bacteria were unable to adapt even over time.
Regarding the tolerance of Nitrobacter, which convert nitrites into nitrates, to pH fluctuations, the following information is available: Nitrobacter carry out oxidation at the highest rate only at a pH of 8.4 to 9.2. Data from domestic wastewater treatment indicate that optimal oxidation rates were observed in the pH range from 8.4 to 8.6. Experience with nitrifying filters in fish farming indicates that the rate of conversion of nitrite to nitrate is high enough to maintain acceptably low nitrite concentrations at pH 6.5–8.5.
Temperature also affects the rate of nitrification in biological filters. As the temperature decreases, the reaction rate decreases.
Provided that stoichiometric oxygen requirements are met, the rate of nitrification does not depend on oxygen concentration. However, at oxygen concentrations below stoichiometric requirements, the reaction rate decreases rapidly (Fig. 4).
Rice. 4. Dependence of nitrification intensity on stoichiometric oxygen demand.
Based on materials from: Wheaton F. Technical support for aquaculture.
The nuances of working with a biological water purification filter
When interacting with such systems, it should be remembered that they represent real “life”, for the maintenance of which it is necessary to take into account a number of required conditions.
- Strict adherence to temperature conditions is vital for bacteria. Otherwise, they will die and you can forget about effective purification of the aqueous solution.
- Proper humidity levels also need to be taken care of. The optimal rate would be no lower than 60% (but more is better).
- To avoid the death of microorganisms, the presence of a nutrient medium for them should be constantly maintained. They require constant nutrition to develop.
Advantages and disadvantages of biological installations
The pronounced advantages include:
- Low operating cost. The cleaning elements do not need to be changed, since the life cycle of the organisms is approximately 5 years.
- Environmental friendliness of this building. Only substances of natural origin are involved in the filtration process.
- High cleaning efficiency.
- At the end of bacterial activity, there is no need for special disposal of decay products.
- If the filtering installation is created according to all the rules and taking into account the proper conditions, it will no longer be necessary to maintain it.
The disadvantages of biofiltration include:
- Impressive size of treatment structures. For the typical average person, accustomed to seeing filters as compact and easily fitting into a small compartment under the sink, this is somewhat shocking information. It will not be possible to place such an installation in a small room.
- The biofilter acts as a secondary stage of purification. It does not rid the liquid of chemical components. Therefore, the aqueous solution should first be filtered to remove them.
For many years, our company has been developing, implementing and producing the most modern water treatment systems. In addition, we provide installation of devices and their warranty service. Consistently high quality products and relatively low prices invariably attract buyers who respond to our work exclusively in a positive way.
At the production base of our company, we carry out a full cycle of manufacturing technical means of cleaning and disinfection of our own design for drinking liquids, as well as assembling devices from components from leading foreign manufacturers.
What affects biological filtration
Biosubstrate area
For several years after biological filtration became commonplace in home aquariums, it was believed that the more porous the material, the more bacteria could settle on it. This is true, but only up to a certain point. The most porous materials with microscopic pores are poorly suited for colonization by bacteria, since for bacterial colonies such sizes are simply small, there is almost no flow in the pores, and the inside of such material is sealed in vain. This does not mean that bioballs are as effective as bioceramics, but one should not assume that the area of the substrate is the main parameter of its quality, especially when we are talking about numbers greater than 500 m2 per liter of substrate.
Biosubstrate volume
The required volume of biosubstrate depends on the volume of the aquarium and the load on the filtration system. Despite the fact that some manufacturers indicate fairly large parameters for their biosubstrates, you need to understand that this is a recommendation most likely for an aquarium that lives well without biological filtration. In practice, especially in fish aquariums, there is not only a much greater load on the filter, but also surges in load, which the “standard” amount of biosubstrate may not be able to cope with. Accordingly, when choosing a filter, especially if it will be the only one, it makes sense to take not the model recommended for your volume, but a larger one.
Volume of pumped water and oxygen saturation
It has been experimentally confirmed that the volume of pumped water does not affect the quality of biological filtration. After achieving sufficient flow, the rate of nitrogen cycle processes continues to be affected only by the saturation of water with oxygen. Any modern external filter provides sufficient flow for biological filtration. Since the effectiveness of biological filtration directly depends on the amount of dissolved oxygen in the water. Good aeration is one of the main prerequisites for good biofilter performance. In planted aquariums, where aeration during the day is not desirable, it is highly desirable to provide it at night.
Ventilation
Purification plants can be supplied with air artificially or naturally. How exactly oxygen is supplied to the system depends on the climate of the area and the type of filter device.
To prevent the structure from being exposed to hypothermia in winter, anti-wind protection is installed next to it. It may look like a dome-shaped fence. In addition, the uniformity of the flow of waste streams is maintained in the filtered liquid. At the same time, the possibility of penetration of cooled air currents to structural elements is significantly limited. To do this, shutters and fabric screens are installed on the ventilation grilles.
The presence of air in the system is also affected by the thickness of the biological membrane. If it grows too much, oxygen supply is cut off. As a result, the active process of decay of dead microorganisms begins - rotting. This problem is especially relevant for drip devices.
Launch
The process of colonizing a biological filter is called starting or cycling.
Bacteria on the substrate, under suitable conditions, appear on their own during the start-up of the aquarium or after adding a new filter to the system. This process may take several days.
To speed up the process, you can add a preparation to the water of a new aquarium to start the aquarium, which should ensure the appearance and nutrition of a colony of bacteria. In addition, the launch accelerates when food for bacteria appears: for example, a pinch of dry food. A good way to speed up the colonization of the biofilter is to add water or soil from a working aquarium.
You can cycle the filter using an already running aquarium and even a bucket or tank of water.